Hodgkin’s lymphoma represents an important health issue, because it has a peak incidence in adolescents, especially the nodular sclerosing subtype. Managing the Hodgkin’s lymphoma during pregnancy is a real challenge, mainly when it is diagnosed in an adolescent woman. The signs and symptoms can be easily confused with symptoms associated to pregnancy. Although the lymph node biopsy provides the certain diagnosis, all the other investigations must be performed. The treatment should be adjusted to each case.
Managementul ante-, intra- şi post-partum al unei adolescente gravide diagnosticate cu limfom Hodgkin
Ante-, intra- and postpartum management of a pregnant adolescent diagnosed with Hodgkin’s lymphoma
First published: 20 septembrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.25.3.2019.2492
Abstract
Rezumat
Limfomul Hodgkin este o reală problemă de sănătate, deoarece incidenţa sa atinge apogeul în perioada adolescenţei, în special subtipul clasic cu scleroză nodulară. Astfel, managementul acestei patologii reprezintă o provocare, mai ales când afectează adolescente. Semnele şi simptomele pot fi cu uşurinţă confundate cu manifestări ale sarcinii. Deşi biopsia ganglionară furnizează diagnosticul de certitudine, este necesară o investigare paraclinică suplimentară. Tratamentul trebuie ajustat fiecărui caz.
Introduction
Hodgkin’s lymphoma (HL) appears when developing lymphocytes suffer a malignant change and multiply in an uncontrolled way. These abnormal lymphocytes – lymphoma cells – form tumors in lymph nodes or in other parts of the body like spleen, liver or bone marrow(1).
HL is one of the most common lymphomas diagnosed during pregnancy, especially because the peak incidence of this disease coincides with female reproductive age(2). HL is diagnosed in approximately 1:1000 to 1:6000 pregnancies(3).
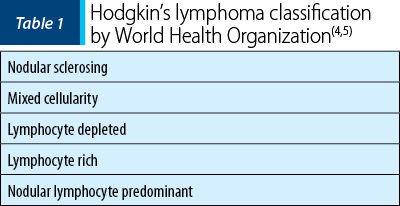
Depending on the histological subtype, the incidence of HL varies in different percentages all over the world: in developed countries it predominates the nodular sclerosis HL accounting in young adults; in economically disadvantaged areas it is more frequent a mixed cellularity HL accounting in children and older adults(4-7).
Pregnant patients with HL have the same signs and symptoms as non-pregnant patients with HL. The particularity of the diagnosis in pregnancy resides in the fact that some signs and symptoms caused by HL (fatigue, shortness of breath, anemia, thrombocytopenia) overlap with common signs and symptoms seen during pregnancy, so it is very possible to omit the correct diagnosis from the beginning and this can cause a delay of diagnosis and precious time is lost(8-10). Anemia can be normochromic/normocytic; microcytic with low iron, but normal or elevated ferritin(8-10). A relatively common finding is eosinophilia that may be determined by the production of chemokines, such as interleukin-5 and eotaxin(8,11,12).
Other signs and symptoms of HL include: asymptomatic lymphadenopathy, unexplained weight loss, fever, night sweats, chest pain, cough, pruritus, palpable, painless lymphadenopathy in the cervical area, axilla, or inguinal area, splenomegaly and/or hepatomegaly(13).
Staging during pregnancy
-
The initial staging evaluation of pregnant patients suspected for HL should include:
-
History and physical examination.
-
Evaluation for supra-diaphragmatic disease which includes a single posterior/anterior view chest X-ray with adequate abdominal shielding, computerized tomography or MRI(14).
-
Laboratory studies: complete blood count, erythrocyte sedimentation rate (ESR, which is often significantly elevated during normal pregnancy), serum creatinine concentration, serum liver enzymes, alkaline phosphatase and HIV serology.
-
Evaluation of the intraabdominal disease with magnetic resonance imaging (MRI), computed tomography or ultrasound(15).
-
Bone marrow biopsy.
During pregnancy gallium scan, PET scan or bone scan are not indicated in order to establish the diagnose of HL(16).
The management of HL during pregnancy requires good communication between the haemato-oncologist, obstetrician, nurse specialists, midwives and neonatologists. Although the goal of treatment should remain curative, there still exists some debate regarding the timing of chemotherapy, the curative nature of treatment and the timing of delivery(17).
Case report
We present the case of a 15-year-old Caucasian patient who was admitted in the Department of Obstetrics and Gynecology of the Bucharest University Emergency Hospital transferred from the Department of Pediatric Oncology of the “Prof. Dr. Alexandru Trestioreanu” Oncological Institute in Bucharest for Hodgkin’s lymphoma nodular sclerosis stage II and 30-31 weeks pregnancy. She was gravida 1, para 1. The patient had insignificant personal pathological history. From her parents, we found out that she was the fourth child, with a normal somatic and neurologic development. She was vaccinated according to the National Program of Vaccination. She had a body mass index (BMI) of 20.3, she was non-smoker and non-hypertensive.
The patient began investigating her pregnancy at 8-9 weeks of amenorrhea. Until 19 weeks of pregnancy, there were no pathological problems, except for a chronic anemia, treated with oral iron. At 19 weeks, she was diagnosed with chronic bronchitis and she was prescribed an antibiotic (ampicillin/sulbactam) for 10 days.
At 24-25 weeks of pregnancy, the previous symptoms were aggravated and she presented to her physician with productive cough, dyspnea and bilateral cervical lymphadenopathy. At the pulmonary exam, bronchi rales were detected, therefore she was directed to the Emergency Room of the Emergency Clinical Hospital of Târgu-Mureş. An ultrasound of soft tissue was performed, which indicated multiple lymphadenopathy – bilateral cervical, bilateral supraclavicular, between 5 mm and 4 cm. She was hospitalized for three days in the Pediatric Department of the Emergency Clinical Hospital of Târgu-Mureş. The blood samples indicated an important inflammatory syndrome, chronic anemia (HGB 7.3 g/dL), an increased LDH and neutrophilia. An MRI was performed and revealed an adenopathyc mediastinal block with large vessels imbedded, with a mass effect on the trachea and esophagus and compressive effect on the superior vena cava and brachiocephalic trunks, confluent adenopathy mass retro and supraclavicular bilateral, axillary bilateral and middle and lower carotid-jugular. A thoracic examination was performed which revealed non-homogeneous pulmonary condensation in the right costo-diaphragmatic sinus without tumor imaging. Also, the intradermal test for Koch bacillus (BK) was effectuated, and sputum examination for BK which was negative.
The patient was transferred afterwards to the Department of Pediatric Oncology of “Prof. Dr. Alexandru Trestioreanu” Oncological Institute from Bucharest. During this hospitalization, the previous signs and symptoms were persistent. The anemia was more pronounced (HGB 7 g/dL). In addition, a thoracic examination was conducted and revealed a mediastinal compression syndrome. The serologies for hepatitis B, C, Epstein-Barr virus, HIV and syphilis were negative, while IgG antibodies for CMV and Toxoplasma were positive. A bone marrow cytology was performed – normal values were observed. A lymph node biopsy from the left cervical adenopathy was performed and it confirmed the certain diagnosis: nodular sclerosis classic Hodgkin’s lymphoma. Corticotherapy was initiated immediately: 8 mg of dexamethasone during the first day, 12 mg per day for the next 10 days, with progressive dose reduction.
After finishing the corticotherapy, the chemotherapy was started, with the agreement of one of the parents – vinblastine 6 mg/m2. The initial evolution was clinically favorable, with diminishing adenopathy. After two weeks, the evolution was not satisfying – right cervical adenopathy increased, the inflammatory syndrome was crescent, so the chemotherapy was changed to ABVD series – doxorubicin 25 mg/m2, bleomycin 5-10 mg/m2, vinblastine 6 mg/m2, dacarbazine 375 mg/m2, all in the first day of treatment. The evolution was good for six days. In the seventh day, the right cervical and supraclavicular adenopathy began to increase in size. A second ABVD chemotherapy series was initiated, with a good clinical tolerance. During the hospitalization, five blood transfusions were performed. The gynecological examination was conducted weekly at the Bucharest University Emergency Hospital and a second-trimester morphology was performed, with normal results, excepting an amniotic fluid index at the inferior limit of the normal range.
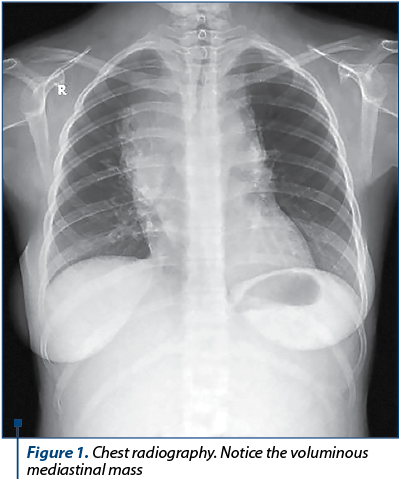
At 30 weeks of pregnancy, the patient was transferred at the Department of Obstetrics and Gynecology of the Bucharest University Emergency Hospital. The obstetrical examination was unremarkable. The ultrasound examination was normal, except for an amniotic fluid index at the inferior limit of normal. The lab tests indicated chronic anemia (HGB 9.9 g/dL). The samples for thrombophilia were analyzed, with no notable modifications. The chest radiography revealed a mediastinal mass (Figure 1). A multidisciplinary team participated in the management of the patient (obstetrician, neonatologist, pediatrician, ENT specialist, hematologist and an intensive care unit specialist). Tocolytic treatment and corticotherapy were initiated. After four days of hospitalization, she delivered by caesarean section – for chronic fetal distress, Hodgkin’s lymphoma in a primiparous patient with an extreme age (15 years old) – a female foetus of 1400 grams, with an Apgar score of 5 at one minute and of 7 at five minutes. The evolution of both the mother and newborn was favorable.
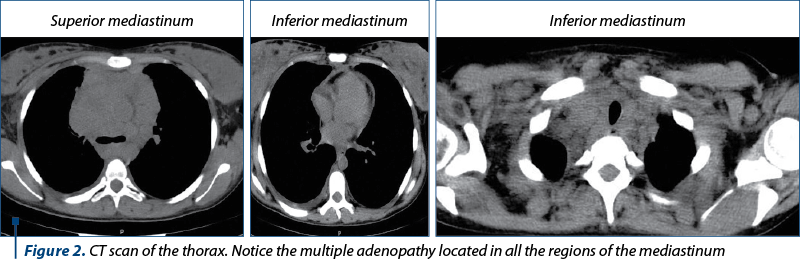
After three days from the caesarean section, a thoracic computed tomography was performed. It detected homogenous multiple adenopathy masses (Figure 2): bilateral supraclavicular lymph nodes of 20 mm, internal jugular vein lymph nodes with an approximately diameter of 37 mm on the right side, prevascular superior mediastinal lymph nodes with a diameter between 20 and 80 mm, Barety lodge deflected the inferior vena cava, in a 65-mm mass continuing with the right tracheobronchial tree, paratracheal lymph nodes having 44 mm on the right side and 22 mm on the left side – diverging the trachea and narrowing its lumen, precarinal and infracarinal lymph nodes having 17 mm, respective 28 mm in diameter, right pulmonary hilum lymph nodes of 43 mm and left pulmonary hilum lymph nodes of 15 mm, left para-aortic lymph nodes of 26 mm, non-specific axillary lymph nodes on the right side of 35 mm and on the left side of 15 mm and supra-diaphragmatic lymph nodes of maximum 14 mm. In addition, interstitial densities in the superior lobes suggesting lymphomatous determinations were detected, areas of osteocondensation suggesting post-radic osteitis, a small pleural effusion, a hepatomegaly and a left hydronephrosis.
After the computed tomography, the mother was transferred to the Department of Pediatric Oncology of the “Prof. Dr. Alexandru Trestioreanu” Oncological Institute, from Bucharest, in order to continue the chemotherapy. At present, the evolution of both the mother and the newborn is favorable.
Discussion
Nodular sclerosis classical Hodgkin’s lymphoma represents 70% of the classic HL in Europe and USA, having a similar incidence in males and females, with peaks at 15-34 years old(18). The chemotherapy during pregnancy is a challenge because it is considered to produce congenital malformations, mutations, carcinogenesis, intrauterine growth restriction and low weight(19). The treatment of early stages is not clearly defined. Some reports suggested that chemotherapy could be deferred during pregnancy or it could be used a single agent, as vinblastine(19-23). In our case, we noticed that the use of a single drug was insufficient and the ABVD regimen was needed in two series.
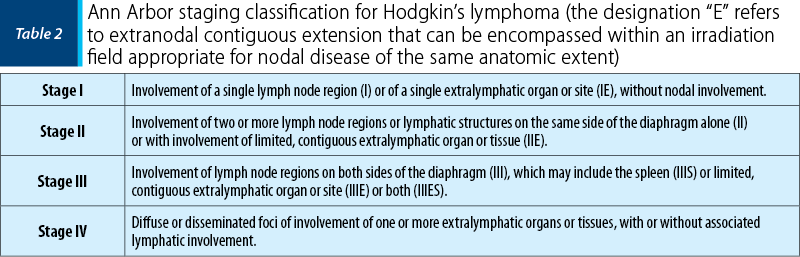
The therapy should be delayed until at least the second trimester, if possible, since the risks associated with the treatment are the greatest for the foetus during the first trimester due to organogenesis. Also, there are some categories of pregnant patients who can start the therapy beyond the first trimester or after delivery – clinical stage IA or IIA HL diagnosed during the late second and third trimester and stable “non-urgent” HL diagnosed after 20 weeks of gestation(24).
The timing of delivery must be a common decision between the obstetrician, hematologist, oncologist and the patient. Caesarean section is not necessary unless the obstetrician indicates this. If the disease is very advanced, the necessary staging laparotomy is performed in conjunction with delivery(24).
Even though the recent studies have shown that the exposure to chemotherapy during pregnancy was not associated with increased central nervous system, cardiac, auditory and other malformations(25), in our case we noticed a low amniotic fluid index and chronic fetal distress which imposed a caesarean extraction of the fetus.
Conclusions
The best management of a pregnant adolescent with stage II Hodgkin’s lymphoma remains chemotherapy. These patients must be treated in multidisciplinary hospitals by an interdisciplinary team.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Woo SY, Fuller LM, Cundiff JH. Radiotherapy during pregnancy for clinical stages IA-IIA Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1992; 23:407.
- Stewart HL Jr, Monto RW. Hodgkin’s disease and pregnancy. Am J Obstet Gynecol. 1952; 63:570-8.
- Lishner M, Zemlickis D, Degendorfer P, et al. Maternal and foetal outcome following Hodgkin’s disease in pregnancy. Br J Cancer. 1992; 65:114-7.
- Aster J, Pozdnayakova O. Epidemiology, pathologic features, and diagnosis of classic Hodgkin lymphoma literature review current through: Feb 2019. This topic last updated: Jun 11, 2018.
- Correa P, O’Conor GT. Epidemiologic patterns of Hodgkin’s disease. Int J Cancer. 1971; 8:192-201.
- Correa P, O’Conor GT. Geographic pathology of lymphoreticular tumors: summary of survey from the geographic pathology committee of the international union against cancer. J Natl Cancer Inst. 1973; 50:1609.
- Gutensohn N, Cole P. Childhood social environment and Hodgkin’s disease. N Engl J Med. 1981; 304:135-40.
- LaCasce A, Ng A. Initial evaluation and diagnosis of classic Hodgkin lymphoma in adults, review current through: Feb 2019. This topic last updated: Jul 07, 2017.
- Shah SJ, Warrier RP, Ode DL, et al. Immune thrombocytopenia and hemolytic anemia associated with Hodgkin disease. J Pediatr Hematol Oncol. 1996; 18:227-9.
- Sierra RD. Coombs-positive hemolytic anemia in Hodgkin’s disease: case presentation and review of the literature. Mil Med. 1991; 156:691-2.
- Di Biagio E, Sánchez-Borges M, Desenne JJ, et al. Eosinophilia in Hodgkin’s disease: a role for interleukin 5. Int Arch Allergy Immunol. 1996; 110:244-51.
- Teruya-Feldstein J, Jaffe ES, Burd PR, et al. Differential chemokine expression in tissues involved by Hodgkin’s disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999; 93:2463-70.
- NCCN Clinical Practice Guidelines in Oncology: Hodgkin Lymphoma. National Comprehensive Cancer Network. Available at: http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. Version 1.2018 — Dec 20, 2017; Accessed: March 22, 2018.
- Levine D, Barnes PD, Edelman RR. Obstetric MR imaging. Radiology. 1999; 211:609-17.
- Pelsang RE. Diagnostic imaging modalities during pregnancy. Obstet Gynecol Clin North Am. 1998; 25:287-300.
- Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. Am J Roentgenol. 2007; 188:1447-74.
- Ansell SM, Armitage JO. Management of Hodgkin lymphoma. In: Mayo Clinic Proceedings. 2006; 81(3):419-26.
- Carbone A, Gloghini A. Nodular sclerosis classical Hodgkin lymphoma (NScHL). Atlas Genet Cytogenet Oncol Haematol. 2017; 21(2):71-5.
- Aviles A, Nambo M, Neri N. Treatment of Early Stages Hodgkin Lymphoma During Pregnancy. Mediterr J Hematol Infect Dis. 2018; 10(1):e2018006.
- Lishner M, Avivi I, Apperley JF, et al. Hematological malignancies in pregnancy. Management guidelines from a international consensus meeting. J Clin Oncol. 2016; 38:501–8.
- Pinnix CC, Andraos, Migrom S, Fanale MA. The management of lymphoma in the setting of pregnancy. Curr Hematol Malig Rep. 2017; 12:251–6.
- Evers AM, Advani R, Press OW, et al. Lymphoma occurring during pregnancy: Antenatal therapy, complications and maternal survival in a multicenter analysis. J Clin Oncol. 2013; 4132–9.
- Eyre TA, Lau IJ, Mackillop L, Collins GP. Management and controversies of classical Hodgkin lymphoma in pregnancy. Br J Haematol. 2015; 169:513–630.
- Jacobs C, Donaldson SS, Rosenberg SA, Kaplan HS. Management of the pregnant patient with Hodgkin’s disease. Ann Intern Med. 1981; 95:669-75.
- Yahalom LA, et al. Management of classic Hodgkin lymphoma during pregnancy. UpToDate. 2007; 534-9.
Articole din ediţiile anterioare
Complicaţiile perinatale şi neonatale la pacientele cu răspuns ovarian scăzut în sarcinile obţinute prin proceduri de reproducere umană asistată
In vitro fertilization (IVF) technologies with a controlled ovarian hyperstimulation approach have classified patients into three different groups ...
Managementul gravidei cu sindrom Sjögren. Prezentare de caz şi review al literaturii
Sindromul Sjögren, o afecţiune cronică ce afectează predominant sexul feminin, este una dintre cele mai frecvente boli autoimune, care însă nu cara...
Ficatul gras acut de sarcină (AFLP). Scurt review şi prezentare de caz
Ficatul gras acut de sarcină (acute fatty liver of pregnancy - AFLP) este o complicaţie obstetricală rară, care apare de obicei în trimestrul al ...
Complicaţiile hipertensiunii arteriale induse de sarcină – management terapeutic şi prognostic
Managementul sarcinii asociate cu hipertensiune gestaţională se realizează în funcţie de severitatea hipertensiunii arteriale, de vârsta ges...