Objectives. Interleukin 1β is a proinflammatory cytokine that plays a critical role in chronic inflammation and carcinogenesis. The aim of this study was to analyze the link between interleukin 1β promoter polymorphism -511 C>T, HPV infection and cervical intraepithelial neoplasia. Material and method. A case-control study was realized. The cases were represented by 128 patients diagnosed with cervical intraepithelial neoplasia, positive at HPV HR testing, while 111 controls, negative for intraepithelial neoplasia and negative at HPV HR testing, were included in the control group. Each person included in the study had a physical examination, cervical cytology, colposcopy, HPV HR testing, DNA extraction from peripheral blood and genotyping for -511 C>T SNP IL 1β using PCR RFLP technique. Results. For the case group, the absolute genotypes frequencies were as follows: 18 - C/C, 52 - C/T, 58 - T/T, respectively for controls: 14 - C/C, 50 - C/T, and 47 - T/T. Chi-square statistics had a value of 0.213, df=1, p=0.644 when the C/T&/T/T genotype was considered as a risk factor, respectively 0.108, df=1, p=0.743 when only homozygous TT genotype was taken into consideration as a risk factor. Odds ration had values of 1.128 (95% CI [0.676 - 1.884]) for C/T&T/T, respectively 0.882 (95% CI [0.417-1.867]) for T/Tgenotype. Conclusions. T/T genotype could be a protective factor for cervical cancer and no statistically significant link has been found between IL 1β-511 C>T and cervical intraepithelial neoplasia.
Variantele genice ale Interleukinei 1β -511 C>T şi neoplazia intraepitelială cervicală
Genetic variants of Interleukin 1-Beta-511 C>T and cervical intraepithelial neoplasia
First published: 15 octombrie 2016
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
Interleukina 1 beta este o cytokină proinflamatorie care joacă un rol important în inflamaţia cronică şi în carcinogeneză. Scopul acestui studiu a fost analizarea legăturii dintre polimorfismul promotorului -511 C>T al interleukinei 1β, infecţia HPV şi neoplazia cervicală intraepitelială. Designul studiului a fost de tip caz-control. Lotul de studiu a fost reprezentat de 128 de paciente diagnosticate cu neoplazie cervicală intraepitelială şi pozitive la testarea HPV, iar lotul de control a fost reprezentat de 111 paciente fără leziuni cervicale şi negative la testarea HPV. Fiecare pacientă inclusă în studiu a fost inclusă într-un protocol unic care a cuprins examenul clinic, examenul citologic Babeş-Papanicolau, colposcopie, testarea HPV, extracţia ADN-ului din sângele periferic şi genotiparea -511 C>T SNP IL 1β utilizând tehnica PCR RFLP. Frecvenţa absolută a genotipurilor pentru grupul de studiu a fost: 18 - C/C, 52 - C/T, 58 - T/T, iar pentru grupul de control a fost: 14 - C/C, 50 - C/T şi 47 - T/T. Testul Chi-square a avut o valoare de 0,213, df =1, cu p =0,644 când genotipul C/T & /T/T a fost considerat ca factor de risc, respectiv 0,108, df =1, cu p =0,743 când genotipul TT homozigot a fost considerat ca factor de risc. Odds ratio au avut valori de 1,128 (95% CI [0,676-1,884]) pentru C/T&T/T, respectiv 0,882 (95%CI [0,417-1,867]) pentru genotipul T/T. Genotipul T/T apare ca un factor de protecţie pentru cancerul de col şi nu s-a constatat nici o legătură statistică între IL 1β -511 C>T şi neoplazia cervicală intraepitelială.
Introduction
Acute inflammatory response represents initially a protective response; nowadays studies bring into discussion the link between chronic infection and neoplasia(1). The mechanisms involved in this transition are extremely complex and only partially understood, immune signal molecules, chemokines, interleukins and the molecules involved in the control of the cellular cycle being involved(1). Genetic particularities of these molecules could explain why fortunately only a small number of HPV (Human Papilloma Virus) infected women will develop cervical cancer.
Interleukin 1b together with interleukin-1a and the antagonist of interleukin 1 belong to the interleukin 1 cytokine family, located on a 430 kb segment on chromosome 2. Interleukin 1b is synthesized mainly by macrophages, but also by other cells of the immune system, with important roles in both acute and chronic inflammatory response(2). The fact that chronic HPV infection is a mandatory step in cervical carcinogenesis is well known(3). Previously published researches confirmed the contribution of interleukin 1b in the neoplastic process by influencing tumor growth and angiogenesis(4), respectively in the induction of chemo resistance(5). Interleukin 1b promoter is polymorphic, multiple SNP (single nucleotide polymorphism) being described at this level (-3737, -1464, -511, -31 and +3954); its genetic variability is the basis of inter-individual differences of the inflammatory response(6).
The first description of IL1b -511 C>T polymorphism was done by di Giovine et al. in 1992(7). While performing a search throughout MEDLINE databases we have encountered 190 articles regarding the association between this polymorphism and human pathologies(8). The presence of this polymorphism was found to be significantly associated with gastroduodenal ulcer(9), neoplasia(10), pulmonary(11) and pancreatic cancer(12). Moreover, the IL1b -511 C>T polymorphism is associated with a high risk of developing sepsis in the context of major trauma(13), puerperal sepsis(14) and even febrile convulsions(15).
This particular genetic polymorphism was proved to be related to gynecological and obstetrical conditions: high risk of preterm labor(16), spontaneous recurrent abortions(17,18), bacterial vaginosis(19), polycystic ovary syndrome(20) and uterine leiomyoma(21). The relationship between this genetic polymorphism and cervical cancer was previously analyzed in two small studies in an Indian and respectively Korean population but not in Caucasians(22,23).
We have chosen IL1b -511 C>T polymorphism for this study due to the association of this particular interleukin with several neoplasia, on one hand, and the importance of promoter region interleukin synthesis and therefore its function, on the other hand. A link between the IL1b -511 C>T polymorphism and cervical intraepithelial neoplasia was investigated on a Romanian population.
Material and methods
Patients’ selection
This study was designed as a longitudinal prospective case-control study and was carried out on a Romanian population between 1st of February 2008 and 30th of June 2010. One hundred and twenty-eight patients diagnosed with intraepithelial neoplasia, positive at HPV HR testing (Atypical Squamous Cells - cannot exclude HSIL - ASC-H: 7; Atypical Squamous Cells of Undetermined Significance - ASCUS: 4; Low-grade Squamous Intraepithelial Lesion - LSIL: 36; High-grade Squamous Intraepithelial Lesion – HSIL: 70; in situ carcinoma - CIS: 11) were included in the case group. One hundred and eleven patients with negatives results for intraepithelial lesion with negative HPV HR tests were included in the control group.
The patients were recruited from the corpus of patients hospitalized or only consulted on an ambulatory base during this period at the First Clinic of Obstetrics and Gynecology, Emergency County Hospital Cluj-Napoca, Romania, willing to participate at this study. The study was approved by the ethical committees and the written consent was obtained for each patient
Biological probes collection
A gynecological examination was performed for each patient. A cervical cytology was obtained. Cervical cytology results were formulated according to Bethesda nomenclature(24). Colposcopic examination was performed for each patient. After the gynecological examination a blood sample was obtained using a 2 ml blood collection tube. The blood samples were immediately stored at 4-8°C.
The analysis of cervical samples
The cervical cytology was examined by a cytologist after a preliminary Papanicolau staining in the laboratory of the above mentioned hospital. HPV HR testing was performed using an immunohistochemistry-based kit.
DNA extraction. Genetic analysis
The genomic DNA was extracted from peripheral leukocytes contained in 300 µl of whole blood using a commercial kit (Wizzard Genomic DNA Purification Kit, Promega®) according to the producer instructions. The extracted DNA was stored in a freezer at -20°C.
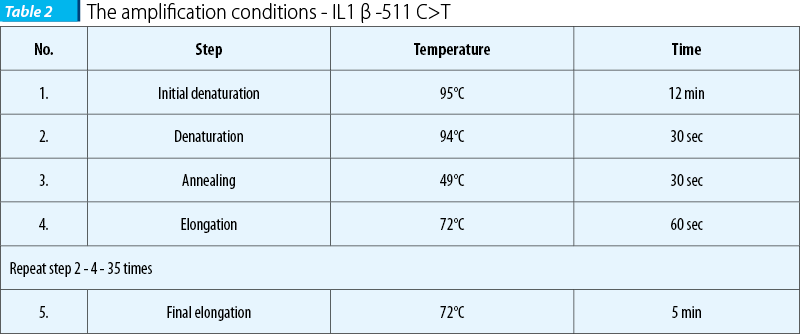
In order to perform the genotyping of IL1b -511 C>T promoter polymorphism, a PCR RFLP technique (Polymerase Chain Reaction - Restriction Fragment Length Polymorphism) was carried out following the protocol described by Ishii et al.(25) and di Giovine et al(7). Briefly, the PCR reaction was done in 25 µl, total volume containing: 12.5 µl 2×PCR mix - Taq DNA polymerase 0.05 U/µl, MgCl2 4 mM, dNTP mix 0.4 mM each (Fermentas MBI, Lithuania®), 1 µl BSA solution (Bovine Serum Albumin, Fermentas MBI, Lithuania®) 2 mg/ml, 1µL from each primer (8 pM), forward and reverse (Eurogentec, Belgium®) (the sequence of the primers is presented in Table 1) µL 75-100 ng genomic DNA. Free nuclease water was added in order to reach the total volume. The PCR protocol is shown in Table 2.

The amplification was followed by enzymatic digestion using 3 UI AvaI® (Fermentas) and 1×Buffer overnight at 37°C. The latter procedure was followed by electrophoresis using a 2% MetaPhor® Agarose (Lonza, Switzerland). The agarose gel was then examined in UV light using a transilluminator (Vilber-Lourmat, France).
Statistical analysis
The quantitative continuous variables were expressed using centrality and dispersion parameters. The qualitative variables were expressed as percent accompanied by the associated 95% confidence intervals(26,27).
Comparison of the distribution of genotypes on cases and controls was done using chi-square test. The differences were considered significant if p-value was lower than 0.05.
Z score was used for comparing two proportions; the level of significance was 5%. CatROM program(28) was used in order to calculate the statistical parameters associated to the 2×2 contingency table for investigation of genetic polymorphisms as risk factor for cervical cancer.
Kolmogorov-Smirnov(29) and Shapiro-Wilks(30) tests were used for testing the normality for experimental quantitative data at a level of significance of 5%.
Data were analyzed with SPSS 16 and the results were summarized using Microsoft Excel 2007.
Results
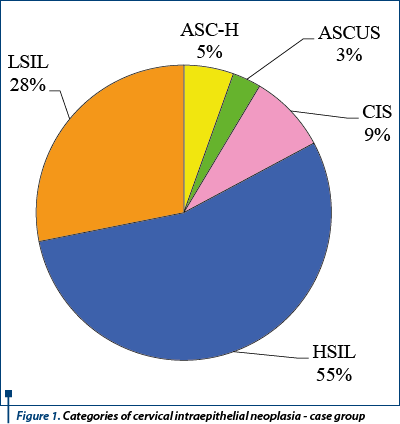
One hundred and twenty eight cases and one hundred and eleven controls were enrolled in this study. Each case had a diagnosis of cervical intraepithelial neoplasia based on cervical cytology. All cases were positive for HPV HR (Human Papilloma Virus - High Risk) based on an immunohistochemical testing of the cervical probe. The most frequent pathology was HSIL (High Grade Squamous Intraepithelial Lesion) - representing 55% (Figure 1). In patients with intraepithelial neoplasia, HSIL is not the most frequent pathology encountered(31); it represents the consequence of the study design.
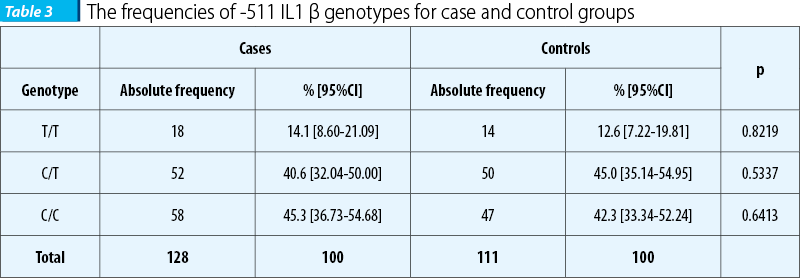
Genotypes distribution is presented in Table 3. The most frequently encountered genotype for cases is C/C homozygous genotype; this genotype is also encountered frequently in controls, but in the latter group the most frequent is the heterozygous genotype (C/T).
Genotypes distribution for each type of cervical intraepithelial neoplasia is presented in Figure 2. The most frequently encountered genotype for controls was the heterozygous genotype C/T; for cases, a similar situation can be observed for ASCUS and ASC-H, while for LSIL and CIS, C/C genotype is the most frequently seen.
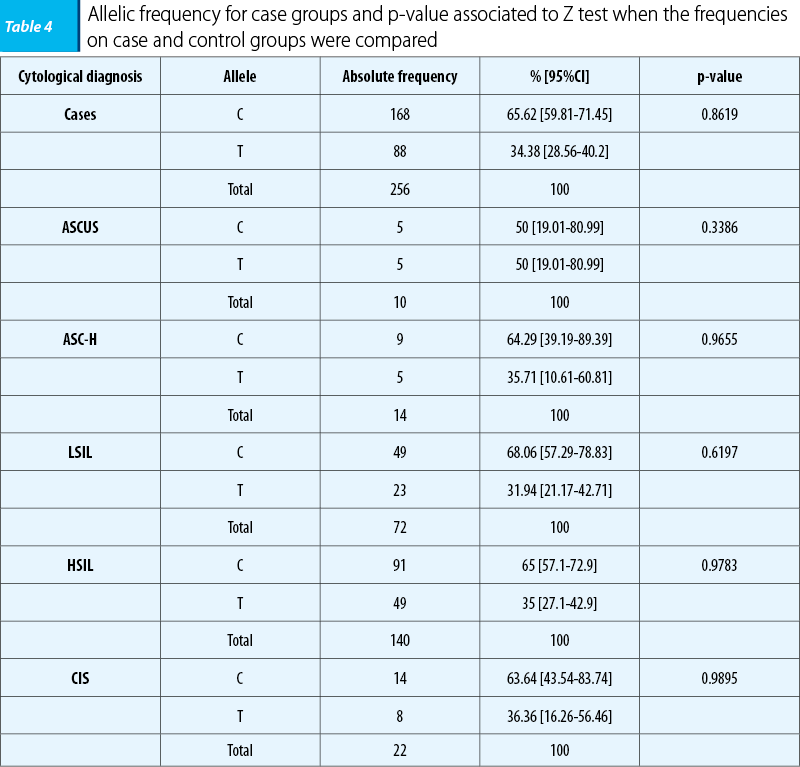

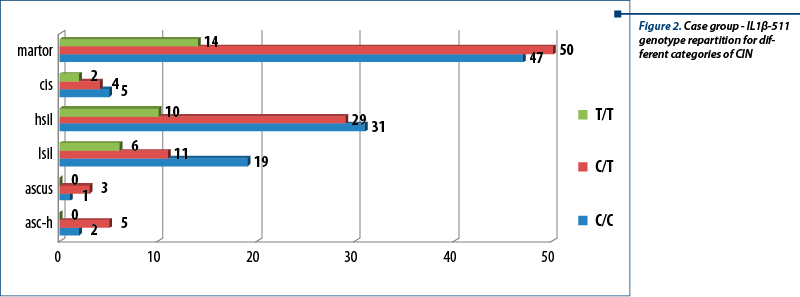
Allele’s frequency was calculated for case and control groups, as well as for each category of cytological diagnosis (for case group). The common allele - C - was the most frequently encountered in both groups and in all categories of cases. The results obtained in analysis on control group are as follow:
-
C: 144 (64.86% [58.58%-71.14%])
-
T: 78 (35.14% [28.86%-41.42%])
-
Total: 222 (100%).
For risk assessment, two hypotheses were analyzed:
-
hypothesis 1: the presence of at least one variant allele is a risk factor (C/T+T/T);
-
hypothesis 2: taking into consideration the homozygous variant genotype (T/T) as a risk factor.
Chi-square and odds ratio were calculated for both hypothesis. The calculated p-values for both hypotheses were done at a significance level of 5%.
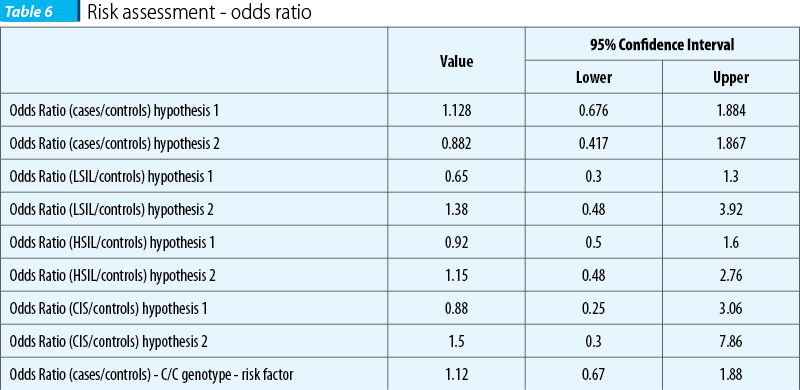
Odds ratios were calculated for both hypotheses, as well as for each category of cytological diagnosis. The results are presented in Table 6.
Discussions
IL-1 b -511 C>T genetic polymorphism seemed to be the perfect candidate as a genetic risk factor for the inflammatory response and cervical cancer. Its localization at the promoter level, the place where several transcription factors acts, has important consequences upon interleukin synthesis(32). The association with cervical cancer was previously identified on an Indian population(22,23). No data were available about the prevalence of this particular polymorphism in the Romanian population on the date when the study was initiated and ended. The present study could not demonstrate a link between cervical intraepithelial neoplasia and the IL-1 b -511 C>T genetic polymorphism. Even if the differences were not statistical significant, the homozygous variant genotype seems to be a protector factor (OR 0.88, 95% CI [0.53-1.48]); similar data had been obtain by Kanget et al(23).
A previous study had investigated the relation between the inflammatory response and IL-1 by measuring the serum level of IL-1 and C reactive protein (CRP)(6). The homozygous-511 C/C genotype proved to be associated with increased levels of IL-1 b(6). Meanwhile, the above mentioned genotype in the presence of C/C genotype at IL1-b -3737 proved to be also correlated with high level of CRP(6). On the other hand, Qian et al.(33) had found significantly higher level of IL-1b in patients with cervical cancer compared to controls. High level of CRP, demonstrated as being a predictive factor for cardiovascular disorders and neoplasia, proved to be associated in cases of cervical cancer with lower survival rates(34). Our data, not significant from the statistical point of view, are providing possible explanation for this link between IL-1 genetic polymorphism, inflammatory response and cervical cancer. Therefore, a raised IL-1 b level linked to -511 C/C genotype seems to be a risk factor for CIN (OR=1.5), while the homozygous variant genotype (T/T) correlated with a low IL-1b seems to be a protective factor (OR=0.88).
Further studies might be necessary on a larger number of patients in order to obtain statically significant results regarding the link between IL1b -511 C>T and CIN. The enlargement of the number of interleukins and chemokines could virtually allow the characterization of the genetic predisposition to develop an inflammatory response. A score system for cardiovascular disease and neoplasia, the major causes of mortality at the beginning of the 21st century, could be elaborated. The present study has also the merit of furnishing data about genotyping -511 IL-1b on a Romanian population.
Conclusions
Among cases, no differences had been observed between different cytological categories, including HSIL and LSIL. Therefore, IL-4 -511 C>T cannot be considered neither favoring, nor protective to the occurrence of cervical neoplasia. No statistical significant differences had been found between the frequencies of different genotypes in case versus control groups. In the present study, the common homozygous genotype seems to be a risk factor for cervical intraepithelial neoplasia, while homozygous variant genotype seems to be a protective factor.
Bibliografie
2. Bird S., Zou J., Wang T. et al. Evolution of interleukin-1beta. Cytokine Growth Factor Rev 2002 13:483-502.
3. Castle P.E., Hillier S.L., Rabe L.K. et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol Biomarkers Prev 2001; 10(10):1021-7.
4. Saijo Y., Tanaka M., Miki M. et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol 2002; 169(1):469-475.
5. Arlt A., Vorndamm J., Muerkoster S. et al. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines, Cancer Res 2002; 62:910-916.
6. Rogus J., Beck J.D., Offenbacher S. et al. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1beta and C-reactive protein. Hum Genet 2008; 123:387-98.
7. di Giovine F.S., Takhsh E., Blakemore A.I. et al. Single base polymorphism at -511 in the human interleukin-1 beta gene (IL1 beta). Hum Mol. Genet 1992; 1:450.
8. NCBI [online] [December 30, 2008] [Accessed September 15, 2015]. Available from: URL: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=16944
9. Cheng, H.H., Chang C.S., Wang H.J. et al. Interleukin-1beta and -10 polymorphisms influence erosive reflux esophagitis and gastritis in Taiwanese patients. J Gastroenterol Hepatol 2010; 25:1443-51.
10. Kumar S., Kumar A., Dixit V.K. Evidences showing association of interleukin-1B polymorphisms with increased risk of gastric cancer in an Indian population. Biochem Biophys Res Commun 2009; 387:456-60.
11. Wu K.S., Zhou X., Zheng F. et al. Influence of interleukin-1 beta genetic polymorphism, smoking and alcohol drinking on the risk of non-small cell lung cancer. Clin Chim Acta, 2010 411:1441-6.
12. Hamacher R., Diersch S., Scheibel M. et al. Interleukin 1 beta gene promoter SNPs are associated with risk of pancreatic cancer. Cytokine 2009 46(2):182-6.
13. Gu W., Zeng L., Zhou et al. Clinical relevance of 13 cytokine gene polymorphisms in Chinese major trauma patients. Intensive Care Med 2010. 36:1261-5.
14. Davis S.M., Clark E.A., Nelson L.T. et al. The association of innate immune response gene polymorphisms and puerperal group A streptococcal sepsis. Am J Obstet Gynecol 2010; 202:308.e1-8.
15. Virta, M., Hurme M., Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol, 2002 26:192-5.
16. Hollegaard M.V., Grove J., Thorsen P. et al. Polymorphisms in the tumor necrosis factor alpha and interleukin 1-beta promoters with possible gene regulatory functions increase the risk of preterm birth. Acta Obstet Gynecol Scand 2008 87:1285-90.
17. Wang Z.C., Hill J.A., Yunis E.J. et al. Maternal CD46H*2 and IL1B-511*1 homozygosity in T helper 1-type immunity to trophoblast antigens in recurrent pregnancy loss. Hum Reprod, 2006, 21:818-22.
18. Hefler L.A., Tempfer C.B., Bashford M.T. et al. Polymorphisms of the angiotensinogen gene, the endothelial nitric oxide synthase gene, and the interleukin-1beta gene promoter in women with idiopathic recurrent miscarriage. Mol Hum Reprod 2002, 8:95-100.
19. Cauci S., Di Santolo M., Casabellata G. et al. Association of interleukin-1beta and interleukin-1 receptor antagonist polymorphisms with bacterial vaginosis in non-pregnant Italian women. Mol Hum Reprod 2007, 13:243-50.
20. Yang Y., Qiao J., Tang R.X. et al. Genotype and haplotype determination of interleukin (IL) 1 beta (g. -511C>T and g. +3954C>T) and IL-1RN in polycystic ovary syndrome. Fertil Steril, 2010 94:384-6.
21. Pietrowski D., Thewes R., Sator M. et al. Uterine leiomyoma is associated with a polymorphism in the interleukin 1-beta gene. Am J Reprod Immunol 2009; 62:112-7.
22. Singh H., Sachan R., Goel H. et al. Genetic variants of interleukin-1RN and interleukin-1beta genes and risk of cervical cancer. BJOG 2008; 115:633-8.
23. Kang S., Kim J.M., Park N.H. et al. Interleukin-1 beta-511 polymorphism and risk of cervical cancer. J Korean Med Sci 2007; 22(1):110-3.
24. Davey, D.D. Cervical cytology classification and the Bethesda System. Cancer J 2003; 9:327-34.
25. Ishii T., Matsuse T., Teramoto S. et al. Neither IL-1beta, IL-1 receptor antagonist, nor TNF-alpha polymorphisms are associated with susceptibility to COPD. Respir Med 2000; 94:847-51.
26. Bolboacă S.D., Achimaş Cadariu A. Binomial Distribution Sample Confidence Intervals Estimation 2. Proportion-like Medical Key Parameters. Leonardo Electronic Journal of Practices and Technologies, 2003; 2-3:75-110.
27. Jäntschi L., Bolboacă S.D. Exact Probabilities and Confidence Limits for Binomial Samples: Applied to the Difference between Two Proportions, The Scientific World Journal, 2010; 10:865-878.
28. Bolboacă S.D., Jäntschi L., Achimaş Cadariu A. Creating Etiology/Prognostic Critical Appraised Topics CATRom Original Software for Romanian Physicians, Applied Medical Informatics, 2003; 13:11-16.
29. Kolmogoroff A. Confidence Limits for an Unknown Distribution Function. Ann Math Statist, 1941; 4:461-463.
30. Shapiro S.S., M.B. Wilk. An analysis of variance test for normality (complete samples). Biometrika 1965; 52:591-611.
31. Rotar I, Mureşan D, Stamatian Fl. Consideraţii actuale privind diagnosticul şi tratamentul neoplaziei intraepiteliale cervicale. O abordare din punctul de vedere al clinicianului. Obstetrica Ginecologia. 2013: LXI: 129-38.
32. Chen H., Wilkins L.M., Aziz N. et al. Single nucleotide polymorphisms in the human interleukin- 1B gene affect transcription according to haplotype context. Hum Mol Genet 2006; 15:519-29.
33. Qian N., Chen X., Han S. et al. Circulating IL-1beta levels, polymorphisms of IL-1B, and risk of cervical cancer in Chinese women. J Cancer Res Clin Oncol 2010; 136:709-16.
34. Wang C.S., C.F. Sun. C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J 2009; 32:471-82.