Antibioticele în aerosoli – ne pot salva?
Aerosolized antibiotics – can they save us?
Abstract
Introduction. In neonatal intensive care units, a serious complication for critical ill patients is represented by ventilator-associated pneumonia (VAP), most often caused by multiresistant Gram-negative bacteria. The treatment of this condition is very challenging, and neonatologists must take into consideration the side effects of broad-spectrum intravenous medications. According to different studies, aerosolized antibiotics could be efficient and safe for treating pulmonary infections in newborns. Case presentation. We report three cases of two extremely low birth weight (ELBW) premature babies and one late preterm with asphyxia who required long-term ventilatory support and who subsequently developed VAP, with the aggravation of their condition. Cultures from tracheal aspirates identified multiresistant Acinetobacter baumannii, rising issues regarding the therapeutic strategy. Colistin is a drug known to be active on most species of Acinetobacter in vitro, but for small premature babies there are concerns regarding its nephro- and neurotoxicity. Therefore, we chose to treat our patients with inhalatory colistimethate sodium for 14-21 days, given twice a day, as monotherapy in one case and as adjunctive therapy in the others, with clinical and bacteriological resolution. All babies could have been extubated and had a good respiratory outcome. Conclusions. In newborns with multiresistant VAP, aerosolized antibiotics can be beneficial and without systemic effects. The doses and regimens are yet to be standardized, but our experience confirms the studies that demonstrate this is a valid treatment which can be used alone or in association with intravenous antibiotics.Keywords
ventilator-associated pneumonianosocomial pneumonianeonatal pneumoniaaerosolized colistinRezumat
Introducere. În secţiile de terapie intensivă neonatală, o complicaţie de temut pentru pacienţii critici este reprezentată de pneumonia de ventilaţie (VAP), cauzată cel mai adesea de bacterii Gram-negative multirezistente. Tratamentul acestei patologii este o provocare pentru medicul neonatolog, care trebuie să ţină cont şi de reacţiile adverse ale administrării intravenoase de antibiotice cu spectru larg. După unele studii, terapia cu antibiotice în aerosoli s-a dovedit a fi eficientă şi lipsită de riscuri şi, de aceea, ar putea fi utilă în tratamentul pneumoniilor neonatale. Prezentare de caz. Prezentăm trei cazuri de pneumonie de ventilaţie diagnosticată la doi prematuri cu greutate extrem de mică la naştere (ELBW) şi la un prematur târziu cu asfixie, care au necesitat suport respirator îndelungat şi, ulterior, au dezvoltat această complicaţie agravantă. Culturile din aspiratul traheal au evidenţiat Acinetobacter baumannii multirezistent, ridicând probleme în ceea ce priveşte alegerea strategiei terapeutice. Colistin este un medicament considerat activ împotriva celor mai multe specii de Acinetobacter in vitro, dar pentru prematurii mici, nefro- şi neurotoxicitatea raportate ca reacţii adverse pot fi probleme grave. De aceea, am preferat să tratăm pacienţii noştri cu colistimetat de sodiu inhalator, pe o durată de 14-21 de zile, în două prize, ca monoterapie într-un caz şi ca terapie adjuvantă în celelalte două, obţinând în toate cele trei cazuri vindecare clinică şi bacteriologică. Nou-născuţii au putut fi detubaţi şi au avut o evoluţie favorabilă din punct de vedere respirator. Concluzii. La nou-născuţii cu pneumonie de ventilaţie cauzată de germeni Gram-negativi multirezistenţi, antibioterapia în aerosoli poate fi eficientă şi lipsită de efecte sistemice. Dozele şi posologia nu sunt încă standardizate, dar experienţa noastră clinică a confirmat rezultatele pozitive ale studiilor ce demonstrează că acesta este un tratament valid care poate fi utilizat ca monoterapie sau în asociere cu tratamentul intravenos.Cuvinte Cheie
pneumonie de ventilaţiepneumonie nosocomialăpneumonie neonatalăaerosoli cu colistinIntroduction
In neonatal intensive care units (NICU), critically ill patients are subjected to many complications, such as nosocomial infections. Among these, ventilator-associated pneumonia (VAP) is one of the most frequently diagnosed conditions, its timing and gravity being influenced by the balance between bacterial colonization from the environment and innate immune host response. There are measures that can decrease the incidence of VAP, but it is impossible to completely eliminate the risk. VAP is an aggravating condition to an already affected organ and it may be difficult to anticipate. The Center for Disease Control (CDC) defines VAP as a change in endotracheal secretions, a need to increase respiratory support, associated with radiographic changes (diffuse or patchy infiltrates, pleural effusion) and with positive endotracheal specimens with negative blood culture in a patient ventilated for more than 48 hours(1). Most of the identified germs are Gram-negative bacteria – Pseudomonas, Acinetobacter, Klebsiella (70%), with resistance to one or more antibiotics classes(2). The prompt intervention is crucial in order to avoid systemic extension and horizontal transmission. As most of the critical patients are already treated with intravenous antibiotics for their primary condition when VAP is diagnosed, the escalation of treatment to broader spectrum antibiotics is necessary. For a premature baby with impaired renal and metabolic functions, this approach can be harmful, with multiple side effects. Therefore, aerosolized antibiotics were tested for pulmonary tissue penetration in neonates and the result was a high concentration of administered drug found in tracheal aspirate 12 up to 24 hours after the procedure(3). These results support the hypothesis that aerosolized antibiotics can be a reliable therapy for pneumonia in newborns.
First clinical uses for inhaled antibiotics date from 1940, with aerosolized tobramycin for chronic infections with Pseudomonas aeruginosa in cystic fibrosis patients. Since then, other drugs, such as aminoglycosides, aztreonam and colistin, were tested, with different rates of success(4). Although clinical efficacy was demonstrated, the American FDA (Food and Drug Administration) did not approve the wide-scale use of aerosolized antibiotic, this treatment remaining off-label, a last resource in cases without other solutions. The American Thoracic Surgery and the Infectious Disease Society of America have stated in their guidelines that “aerosolized antibiotics may be considered as adjunctive therapy in patients with multidrug-resistant Gram-negatives who are not responding to systemic therapy”(5). Other authors suggest that aerosolized antibiotics can be also used in the prevention of VAP in patients with multiple risk factors(6). The doses and the regimen differ from a center to another, but the results are positive and encouraging in all reports.
Belonging to polymyxins antibiotic family, colistin is a concentration dependent drug which was first used in medical practice in 1959 and it proved to be efficient against Gram-negative bacteria. Its reported side effects – nephro- and neurotoxicity – represented a serious drawback and in 1970 the drug was excluded from most therapeutic protocols(7). Nevertheless, due to the increased bacterial resistance to many classes of antibiotics in the following years, colistin regained nowadays its importance in clinical practice as a valid solution for the treatment of nosocomial infections.
Colistin is available for medical use as colistimethate sodium or sulphate, but only colistimethate is appropriate for parenteral and aerosolized use, sulphate being recommended orally or topically. Colistimethate is an inactive form of the drug that is spontaneously hydrolyzed at physiological pH to an active metabolite. Its bactericidal activity results from binding to anionic lipopolysaccharides from the bacterial outer membrane, dislocating calcium and magnesium, disrupting membrane continuity and leading to cellular death. It can also neutralize bacterial endotoxins and inflammatory cytokines, blocking their activity, resulting in a supplemental anti-inflammatory effect(7,8).
Case presentations
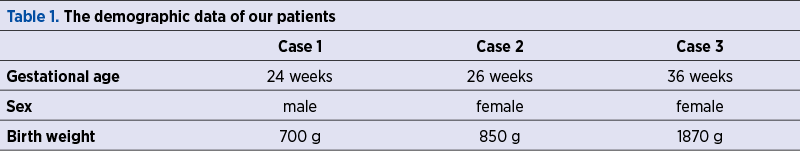
We report three cases of newborns admitted during the last year in the Neonatal Intensive Care Unit of the University Emergency Hospital Bucharest for advanced life support: two extremely low birth weight (ELBW) premature babies with respiratory distress syndrome (RDS) and associated complications of prematurity, and one late preterm infant with birth asphyxia.
All babies were intubated at birth and required long-term respiratory care. The anamnesis revealed maternal history of infections during pregnancy and the laboratory tests showed neonatal positive inflammatory markers in the first 24 hours of life, therefore intravenous antibiotherapy was prescribed. Despite treatment and preventive nursing care, the clinical course was unfavorable, with the impossibility to wean from the ventilator support for more than 14 days. VAP was diagnosed based on the increased respiratory effort, abundant secretions in upper and lower airways, and the need to rise oxygen delivery (FiO2) to maintain optimal oxygen saturation (SaO2). Until bacteriological tests results were available, the patients were given broad-spectrum intravenous antibiotics, with poor clinical response.
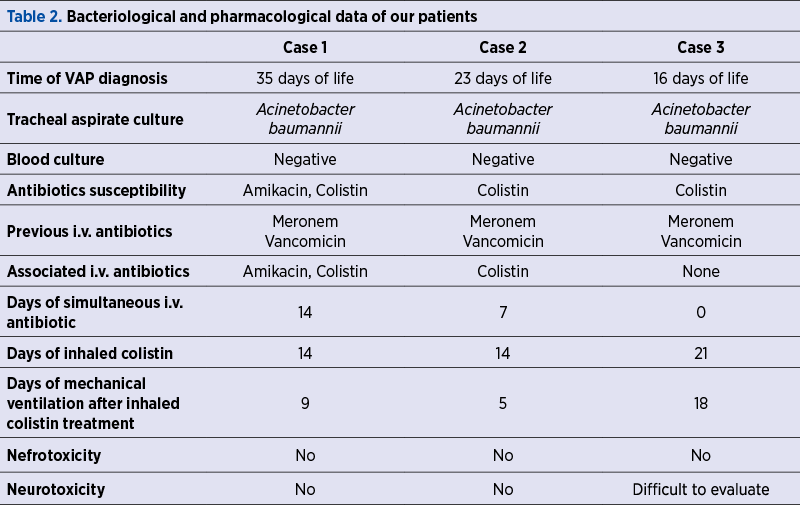
Once tracheal aspirate tested positive for Acinetobacter baumannii, multiresistant, susceptible to colistin only and intermediate to amikacin (in one case); the babies were switched on aerosolized colistin associated with intravenous amikacin and colistin (in the first case), intravenous colistin only (in the second case), and no intravenous antibiotic in the third case because of a previously transient renal failure (probably due to hypoxia). The further course of the disease showed gradual improvement of the respiratory status, allowing FiO2 and pressures to be decreased and making extubation possible after nine, five and, respectively, 18 days. Noninvasive ventilation and free flow oxygen were still necessary for a period of time (two of the children developed chronic lung disease), but the inflammatory response and cultures became negative. We did not observe any alteration of the neurological status compared to the initial evaluation and the renal function was normal in all three cases during the treatment. No other adverse reactions were noted.
Disscussion
Despite the reported benefits, the clinical use of inhaled colistin for newborns – especially preterm – is not widely used due to insufficient evidence-based data. Every NICU should develop and follow a local protocol based on clinical, paraclinical and bacteriological data and a risk-benefit analysis. When bacterial susceptibility limits the therapeutic options and infection becomes life-threatening, colistin is a drug to be used. Although the intravenous administration is generally considered more effective and better controlled, the impaired renal function or neurological depression that are often encountered in premature born babies with respiratory insufficiency can be aggravated by the systemic use of colistin.
Studies review reveals that colistin can be administered as monotherapy or associated with others antibiotics; it can be prescribed both aerosolized and intravenously or just inhaled, with similar results(3,4). When the tracheal and plasma concentration of colistin were measured, the values surpassed minimum inhibitory concentration (CMI) in the airways and were insignificant in the bloodstream(3). These results are encouraging for the use of inhaled administration only, which acts selectively on the pulmonary tissue and avoids the systemic effects.
In our cases, we chose to give only inhaled colistin to one patient with altered excretion (oliguria and high urea and creatinine at admission) and both intravenous and inhaled colistin in the other two cases. In the first case, where the baby was severely affected and the antibiogram showed susceptibility also to amikacin, we associated it intravenously for seven days, with no alteration of renal function.
There are not standardized regimens for the inhaled colistin administration in newborns. Studies reported efficacy in adult patients with a dose of 80 mg colistimethate (CMS) in 3 ml normal saline (NS) every 8-12 hours(8). For newborns, the intravenous doses are 2.5-5 mg/kg/day, in 2-4 doses(7). The inhaled doses used in most studies are 4 mg/kg/dose every 12 hours, administered in 4 ml of normal saline during 15 minutes(3,4). The recommended treatment period is 14 days(3). Our patients were given 5 mg/kg/12 hours for 14 days (two cases) and for 21 days (in the third case), proving that aerosolized colistin as singular treatment may need more time to work.
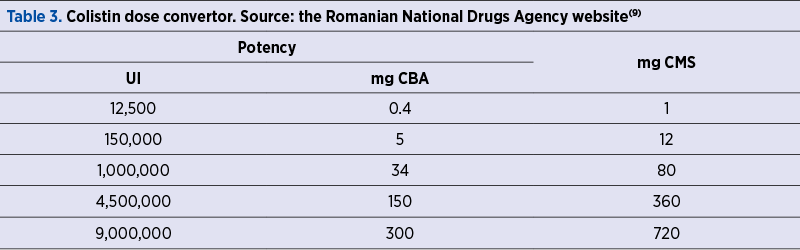
Attention should be paid to conversion from international units (IU) to milligrams (nominal drug potency = 12,500 IU/mg; 1 mg = 12,500 IU), as countries from the European Union labels content in IU, compared to the United States of America, where milligrams of CBA (colistin base activity) are preferred.
Most reported side effects for intravenous colistin use are neurotoxicity (lethargy, sight loss, ataxy, paresthesia, neuromuscular blockade manifested as apnea, respiratory insufficiency) and nephrotoxicity (proteinuria, hematuria, oliguria, tubular acidosis, increased urea and creatinine), rashes, eczema(7). Colistin used in aerosols can cause mild bronchoconstriction which can be avoided if a beta-2 agonist is previously administered(7). We did not experiment any side effects in our patients.
It is important to know what kind of device to use for optimizing inhaled colistin treatment. During mechanical ventilation, particles larger than 3 micrometers remain in the upper respiratory tract, while the smallest particles (less than 0.5 micrometers) diffuse in the air or in the ventilatory circuit, therefore one should use an equipment able to provide right sized inhaled particles that can reach the alveoli (1-3 micrometers). In order to standardize the particles’ size, a constant ventilator flow is required, preferably set on a volume-controlled mode(10). The best aerosol device is a jet nebulizer using air or oxygen at high pressure or a mesh vibrating nebulizer – which we used – easy to adapt to the ventilatory circuit, with the facility to set the particles’ size, thus allowing a very small residual antibiotic volume. There are other types of nebulizers, using ultrasounds. It is demonstrated that the efficacy of nebulization is increased when the device is placed further on the ventilatory circuit, on the inspiratory branch at a distance of 15-40 cm away from the endotracheal tube (ETT)(8,10), although good results were also reported when a connection with inspiratory line was made at 5 cm from the “Y” piece(3). Humidifier and flow sensor should be excluded during nebulization.
Studies have shown that, although there is a lower penetration of antibiotic in the condensed areas compared with well-ventilated areas, and drug concentration is lower than in the tracheal aspirate, there is still a higher alveolar penetration obtained through nebulization than if the antibiotics were administered intravenously(11). If infection becomes systemic and affects the central nervous system, nebulized colistin should be doubled by the intravenous route. Even in these cases, the microbiological cure has been obtained in 94.2% of patients and the survival rate was 76.6%(12).
Conclusions
VAP is encountered in 25-50% of pediatric intensive care patients(13) or in 6.5 cases/1000 ventilator days(14), generating a mortality rate of 30-60%(15,16) and other poor outcomes such as prolonged mechanical ventilation, extended hospital stay, or alteration in neurological and nutritional status. As it is not possible to completely avoid this potentially fatal complication and the antibiotics resistance has been alarmingly rising in the last years, alternative interventions that could help overcome this complication and which are safe and efficient have become of great interest for the clinicians. Aerosolized antibiotics can sometimes save us and our patients, being an affordable and available solution that can be administered to all NICU patients, without risks or special skills.
Financial support: none declared.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
https://www.cdc.gov/infectioncontrol/guidelines/mdro/introduction.html
-
Borgatta B, Rello J. How to approach and treat VAP in ICU patients. BMC Infect Dis. 2014 Apr 30;14:211. doi: 10.1186/1471-2334-14-211.
-
Nakwan N, Lertpichaluk P, Chokephaibulkit K, Villani P, Regazzi M, Imberti R. Pulmonary and Systemic Pharmacokinetics of Colistin Following a Single Dose of Nebulized Colistimethate in Mechanically Ventilated Neonates. Pediatr Infect Dis J. 2015;34(9):961-963. doi:10.1097/INF.0000000000000775.
-
Xu F, He LL, Che LQ, Li W, Ying SM, Chen ZH, Shen HH. Aerosolized antibiotics for ventilator-associated pneumonia: a pairwise and Bayesian network meta-analysis. Crit Care. 2018 Nov 15;22(1):301. doi:10.1186/s13054-018-2106-x.
-
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. doi:10.1164/rccm.200405-644ST.
-
Falagas ME, Siempos II, Bliziotis IA, Michalopoulos A. Administration of antibiotics via the respiratory tract for the prevention of ICU-acquired pneumonia: a meta-analysis of comparative trials. Crit Care. 2006;10(4):R123. doi:10.1186/cc5032.
-
Loho T, Dharmayanti A. Colistin: an antibiotic and its role in multiresistant Gram-negative infections. Acta Med Indones. 2015 Apr;47(2):157-68.
-
Antoniu SA, Cojocaru I. Inhaled colistin for lower respiratory tract infections. Expert Opin Drug Deliv. 2012 Mar;9(3):333-42. doi: 10.1517/17425247.2012.660480.
-
https://www.anm.ro/_/_RCP/RCP_7451_ 27.02.15.pdf
-
Quon BS, Goss CH, Ramsey BW. Inhaled antibiotics for lower airway infections. Ann Am Thorac Soc. 2014 Mar;11(3):425-34. doi:10.1513/AnnalsATS.201311-395FR.
-
Goldstein I, Wallet F, Nicolas-Robin A, et al. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med. 2002;166(10):1375–81.
-
Nakwan N, Chokephaibulkit K, Imberti R. The Use of Colistin for the Treatment of Multidrug-resistant Gram-negative Infections in Neonates and Infants: A Review of the Literature. Pediatr Infect Dis J. 2019;38(11):1107-1112. doi:10.1097/INF.0000000000002448.
-
Kang CH, Tsai CM, Wu TH, et al. Colistin inhalation monotherapy for ventilator-associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr Pulmonol. 2014;49(4):381-388. doi:10.1002/ppul.22750.
-
Martin RJ, Avroy AF, Walsh MC. Fanaroff & Martin’s Neonatal-Perinatal Medicine, Diseases of the fetus and Infant, 10th edition, Complications of Assisted Ventilation, Elsevier, Saunders, 2015, 1105-1106.
-
Petdachai W. Ventilator-associated pneumonia in a newborn intensive care unit. Southeast Asian J Trop Med Public Health. 2004;35(3):724-729.
-
Garland JS. Strategies to prevent ventilator-associated pneumonia in neonates. Clin Perinatol. 2010;37(3):629-643. doi:10.1016/j.clp.2010.05.003.




