Caesarean scar pregnancy (CSP) is a potential complication of women having pregnancies after one or more previous caesarean deliveries. Its incidence varies between 1:1800 and 1:2200 pregnancies. The most important aspect is the diagnosis of CSP as early as possible, ideally around 7 weeks of gestation. The failure to diagnose CSP could lead to a high risk of uncontrolled bleeding and caesarean hysterectomy, with increased morbidity and mortality for both mother and fetus. Many management strategies have been presented, ranging from medical to surgical interventions or combining them. We recommend the use of Shirodkar cervical suture (to control the potential bleeding) preceding transcervical suction curettage of pregnancy.
Sarcina implantată pe cicatricea operaţiei cezariene – management
Caesarean scar pregnancy – management
First published: 16 martie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.68.1.2020.3028
Abstract
Rezumat
Sarcina implantată pe cicatricea operaţiei cezariene este o complicaţie potenţială a sarcinii survenite la o femeie cu mai multe naşteri prin operaţia cezariană. Incidenţa sa variază între 1:1800 şi 1:2200 de sarcini. Cel mai important aspect este ca diagnosticul sarcinii implantate pe cicatricea de cezariană (SICC) să fie stabilit cât mai repede posibil, ideal la 7 săptămâni de gestaţie. Eşecul diagnosticului poate duce la un risc crescut de hemoragie masivă şi de histerectomie postcezariană, cu un risc crescut al morbidităţii şi mortalităţii, atât materne, cât şi fetale. Au fost prezentate mai multe strategii de conduită terapeutică, de la cele medicale la cele chirurgicale sau combinarea lor. Autorii recomandă folosirea cerclajului cervical Shirodkar (pentru a controla hemoragia potenţială), urmată de chiuretajul aspirativ al sarcinii.
Counseling the patient
Once the diagnosis of caesarean scar pregnancy (CSP) was established, the patient must be informed and counseled about her therapeutic options. In the presence of a live CSP proven by ultrasound and b-hCG, the patient must be informed that an immediate decision has to be taken in order to prevent the further growth of the embryo.
The patient and her partner should clearly understand that the pregnancy could evolve until term with the delivery of a live offspring, but the possibility to have an associated complication, such as the uterine rupture before fetal viability or morbidly adherent placenta, is very high and the risk of significant morbidity (heavy bleeding, massive transfusion, emergency caesarean hysterectomy) and mortality is present(1). The highest number of patients will opt for the termination of pregnancy and very few will assume to continue.
Management methods
Some of the caesarean scar pregnancies will be nonviable pregnancies, and they will end through spontaneous miscarriage (40%)(2).
There is a myriad of medical and surgical procedures, most of them combined, recommended for such a diagnosis. None of them is reported to be certainly successful. The method(s) recommended depends, firstly, on the experience of the team and the protocol of the institution. It is of outmost importance that such a diagnosis be certain and the patient be referred to an institution with all required resources and team experience to deal with such cases.
The treatment should be individualized, according to the patient’s age, number of previous caesarean deliveries, number of children, and the expertise of the institutional team to manage such cases(3).
The medical treatment uses methotrexate (MTX), systemic or injected into the gestational sac. It was used as first-line, single i.m. dose, of 1 mg/kg body weight or 50 mg/m2 of the body surface area(1). The injection of MTX into the gestational sac is performed with abdominal or transvaginal guidance. Systemic single-dose of MTX is associated with a complication rate of 64.6%, mostly because the failure of the first treatment requires the second administration of the drug(1).
The outcome of the treatment with MTX is controversial. Its slow action or failure to stop the cardiac activity requires to repeat the dose or second-line treatment. As a result of repeating the dose, two-thirds of the treated patients will develop complications (liver and bone marrow). During the days of waiting, the embryo or fetus and its vascularity can grow, making more difficult the treatment and increasing the rate of serious morbidity and even mortality(3).
Minimally invasive procedures involve ultrasound-guided local injection of MTX or potassium chloride into the gestational sac.
Timor-Tritsch et al., in a review of 750 cases published in the literature, mentioned a rate of complications of 10.8% for transvaginal route and a higher rate, of 15%, for abdominal ultrasound guidance(1).
Some protocols recommend the association of MTX with uterine artery embolization performed by the interventional radiologist under general anesthesia. The complication rate of the procedure was 47% of the 64 cases reported in literature(3).
Some authors argue that MTX, systemically or in local injection, could be recommended when future fertility is desired(3).
Surgical procedures recognize open or laparoscopic excision of bulging gestational sac, suction aspiration, dilatation and curettage (D&C), and hysteroscopic resection.
The insertion of a Foley catheter after D&C or suction aspiration is recommended by some authors(4,5).
There is no evidence of any specific intervention over another for the treatment of CSP. Most authors support surgical rather than medical approach as the most successful(6).
We present the technique we used in a case of a first-trimester CSP treated by suction curettage under abdominal ultrasound-guided continuous examination, combined with the placement of a Shirodkar cerclage suture in order to stop a possible post-evacuation heavy bleeding.
The diagnosis of viable CSP of 10 weeks was confirmed by b-hCG and transvaginal ultrasound. The patient was informed and consent for surgical evacuation, before placing a Shirodkar cerclage suture.
The surgical evacuation was performed under general anesthesia. The patient received a prophylactic dose of 1.5 g of cefuroxime before starting anesthesia. After cleansing the vulva and vagina, the uterine cervix was visualized, two Pozzi forceps were placed at 3 and 9 o’clock of the cervix and used for traction (Figure 1).
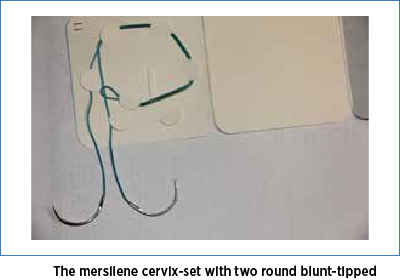
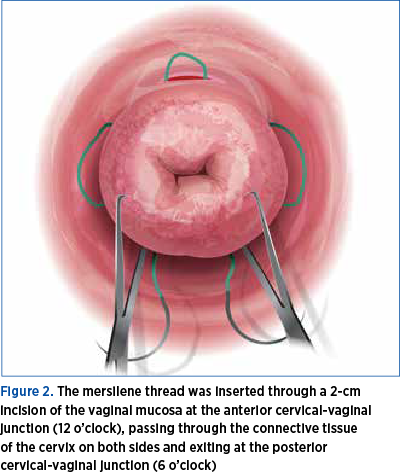
The vaginal mucosa, at the level of its junction with the uterine cervix, was infiltrated with saline solution in order to facilitate hydro-dissection. A 2 cm long transverse incision was made at the level of the anterior junction of vaginal mucosa with cervix uteri. The incision interested the full thickness of the vaginal mucosa, giving access to the supravaginal portion of the uterine cervix. The bladder was dissected and pushed upward. A mersilene cervix-set (Figure 2), with two round blunt-tipped needles, was used to perform the cervical cerclage. The mersilene thread is placed starting from 12 o’clock, passing through the uterine cervix under the vaginal mucosa, to the right side of the cervix, exiting to the surface of vaginal mucosa at 9 o’clock, reentering under the vaginal mucosa to exit at 6 o’clock.
The same maneuver is performed with the second needle to the left side of the cervix, starting at 12 o’clock, passing under vaginal mucosa and exiting at 3 o’clock to re-enter at this level, passing under the vaginal mucosa and exiting at 6 o’clock, as high as possible, at the level of posterior cul-de-sac of vagina. The needles are cut and the ends of the thread are left untied, caught with a small forceps(7-10).
It is important that the needle pass through the dense collagen layer of the cervical stroma, to avoid slippery of the thread and its failure (Figure 3).
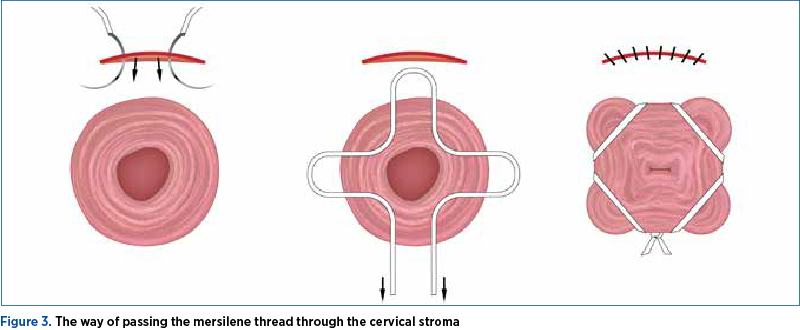
We don’t recommend a second incision of the vaginal mucosa on the posterior aspect of the cervix for the last step of the cerclage suture.
Placing the knot on the back of the cervix, at 6 o’clock, one can reach the highest point close to the internal os of the cervix. Putting the suture here and finally removing the knot is associated with less risk of injuring the rectum(11).
From a technical point of view, the better exposure of the surgical field can be reached by using a weighted speculum to retract the posterior vaginal wall, and two Breisky right-angle retractors to retract the bladder and expose the cervix.
The next step is the dilatation of the uterine cervix in order to prepare suction curettage. The dilatation is performed usually using, progressively, Hégar dilators. The size of the cannula depends on the size of pregnancy. Cervical scar pregnancy is evacuated using suction curettage under continuous transabdominal ultrasound guidance(12) (Figure 4).
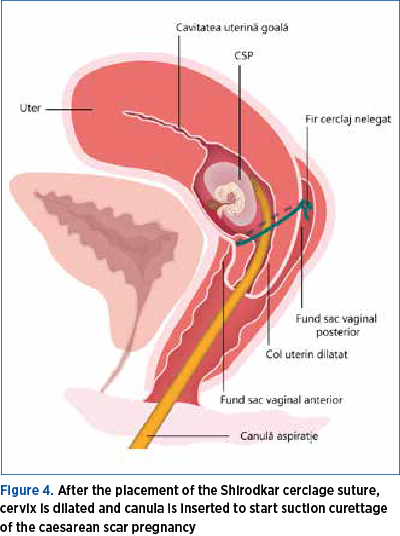
Ultrasound guidance is indispensable for the safety of the procedure, decreasing the risk of uterine perforation. It also helps the gynecologist to complete removal of placental tissue. In case of obese patients, transrectal ultrasound could be an option.
Once the abdominal ultrasound showed that pregnancy has been completely removed, blood loss is estimated. The blood loss is assessed by estimating the bleeding through the cervical os, the size of hematometra and the blood accumulated in the pouch of Douglas, both by transvaginal ultrasound examination. In case of major or continuous bleeding, the gynecologist has the option to tie the cervical suture in order to obstruct the cervical canal, leading to the formation of a hematometra that will facilitate the tamponade of the placental site and eventually will stop the bleeding(12).
It was shown that introducing a Foley catheter into the remaining cavity of the aspirated cervical scar pregnancy one could stop bleeding(4). This can be an option in case of growing hematometra.
The surgical measures to stop the bleeding might be empowered by medical measures (ergometrine 500 µg, i.m.)(12).
It was shown that Doppler examination before suction curettage is useful to predict excessive blood loss(13).
The patient is kept under anesthesia until complete hemostasis is achieved: no external bleeding through the cervical os, stable hematometra, and no blood in the pouch of Douglas. If this happens, cervical cerclage is removed and anesthesia is stopped(12).
If bleeding was less than 1000 ml, hemostasis is controlled, there is no anemia and the patient is in a good clinical shape, she can be discharged in the same afternoon. If the patient has the cervical cerclage tied and is stable, she could also be discharged or stay until the next day when the suture will be removed(12). The cerclage suture should be kept in place no more than 24 hours. For patients discharged from the hospital with suture in place, Jurcovic et al. recommend prophylactic antibiotics (amoxiclav, 625 mg x 3/day).
If the patient was discharged, she is invited to return to the hospital in the next 2-7 days for a transvaginal ultrasound in order to exclude retained trophoblastic/placental tissue.
At 6-8 weeks after CSP evacuation, the patient should be examined by transvaginal ultrasound in order to evaluate the myometrial defect and answer the questions about contraception or future fertility(12).
At two months postoperatively, the patient was invited for a transvaginal ultrasound that showed a thin layer of the anterior myometrium and caesarean scar defined as a triangular anechoic structure giving the aspect of niche or sacculation (Figure 5).
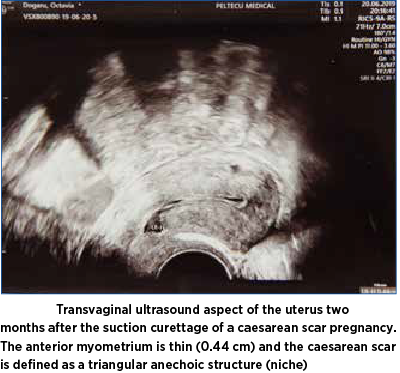
Discussion
Any suspicion of CSP must be sent to a tertiary center of obstetrics and gynecology in order to clarify the diagnosis and take the best decision.
Most authors recommend screening in early pregnancy for CSP of all pregnant patients with a history of caesarean section(14,15).
There is a myriad of treatments, ranging from medical (MTX) to mini-invasive (MTX or potassium chloride local injection, laparoscopy, hysteroscopy, uterine artery embolization, suction curettage), open surgery or combinations (MTZ or KCl and suction curettage; MTX and UAE; Shirodkar cerclage and suction aspiration, Foley catheter).
Most authors agree that the surgical treatment is more successful than the medical one, and that the combination is more successful than the single treatment.
All these treatments are recommended for CSP in the first trimester. Some reports estimate that about 44% of the CSP will end as spontaneous miscarriage in the first 10 weeks of gestation(16). The best moment to treat CSP is between 7 and 9 weeks of gestation(2).
Conclusions
The natural history of CSP is poorly understood. Counseling a patient with a confirmed CSP is crucial. The patient’s option to continue pregnancy is associated with a high risk of placenta accreta spectrum syndrome, with severe hemorrhage, massive transfusion and with caesarean hysterectomy.
The earlier the diagnosis of CSP, the less complications are, and more time to discuss with the patient and take decisions. The complications are related to misdiagnosis or late diagnosis.
Most of the authors support the surgical than the medical approach as the most successful.
Suction curettage associated with prophylactic cerclage represents a simple, safe and effective procedure.
There is no proven benefit of the niche revision after the surgical evacuation of CSP.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Zosmer N, Fuller J, Shaik KH, et al. Natural history of early first-trimester pregnancies implanted in caesarean scar. Ultrasound Obstet Gynecol. 2015; 46:367-75.
3. Timor-Tritsch IE, Monteagudo A, Agten AK. Caesarean scar pregnancy, diagnosis and management. Contemporary Obstet Gynecol. 2015; nr 24.
4. Timor-Trisch IE, Monteagudo A, Santo R, et al. The treatment and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012; 207(1):44.e1-e.13.
5. Jiang T, Lui G, Huang L, et al. Methotrexate therapy followed by suction curettage, followed by Foley catheter for tamponade after cesarean scar pregnancy. Eur J Obstet Gynecol Biol. 2011; 156(2):209-211.
6. Diagnosis and management of ectopic pregnancy. Green-top Guideline No. 21. BJOG. 2016; 123.e15-e.55.
7. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955; 52:299.
8. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957; 64(3):346-350.
9. Caspi E, Schneider DF, Mor Z, et al. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Obstet Gynecol. 1990; 7(4):347-350.
10. Mashiach S, Admon D, Oelsner G, et al. Cervical Shirodkar cerclage may be the treatment of choice for cervical pregnancy. Hum Reprod. 2002; 17:493-496.
11. Katz M, Abrahamson C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005; 192(6):1989-92.
12. Jurcovic D, Knez J, Appiah A, et al. Surgical treatment of Cesarean scar pregnancy: efficacy and safety of ultrasound-guided suction curettage. Ultrasound Obstet Gynecol. 2016; 47:511-517.
13. Akbayir O, Gedikbasi A, Akyal A, et al. Cervical scar pregnancy: a rare cause of uterine arteriovenous malformation. J Clin Ultrasound. 2011; 39:534-538.
14. Gonzales N, Tulandi T. Cesarean scar pregnancy: A systematic review. J Minim Invasive Gynecol. 2017; 24:731-8.
15. Panaitowa J, Tokunaka M, Krajewska K, Zosmer N, Nicolaides KH. Screening for morbidly adherent placenta in early pregnancy. Ultrasound Obstet Gynecol. 2019 Jan; 53(1):101-106.
16. Jurcovic D, Hillaby K, Woelfer B, et al. First trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol. 2003; 21:220-227.
Articole din ediţiile anterioare
Placenta succenturiată – provocări în diagnosticul şi managementul prenatal. Raport de caz
Anomaliile placentei au fost asociate cu o incidenţă mai mare a morbidităţii şi mortalităţii materne şi fetale. Placenta cu lob placentar accesor e...
Omfalocel, scrot bifid, hipospadias şi micropenis: rezultate clinice în cazuri cu cariotip normal
Acest studiu îşi propune să descrie caracteristicile, monitorizarea şi managementul unei malformaţii complexe: omfalocel, hipospadias, scrot bifid ...
Studiu retrospectiv privind avortul precoce, efectuat în cadrul Departamentului de obstetrică şi ginecologie al Spitalului Universitar de Urgenţă Bucureşti
Abortion is the termination of pregnancy before 20 weeks of gestation. Spontaneous abortion or miscarriage is a naturally occurring event, unlike i...
Provocări în evaluarea feţei fetale – diagnostic şi management
The ultrasound assessment of the fetal face is the first way of interaction of the parents with their unborn baby, therefore recent achievements in...