Intracystic (encysted) papillary cancer (IPC) is a rare form of breast neoplasia with an excellent prognosis and a low recurrence rate. It usually occurs in postmenopausal women. Its natural history and proper management are not fully acknowledged. The symptoms are not specific, explaining the late presentation for medical examination. Moreover, routine investigations such as mammography, breast ultrasound or cytology can misdiagnose the IPC. Conservative surgery is reported to be the most efficient, consisting in surgical excision of the lump with negative margins of 2 mm, with or without sentinel lymph node biopsy. Additional therapies are discussed by the multidisciplinary team. We report two cases of IPC and we present a short review of the literature.
Carcinomul mamar intrachistic, o boală rară: prezentare de cazuri şi revizuire a literaturii
Intracystic papillary breast cancer, a rare disease: case reports and literature review
First published: 25 aprilie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.69.2.2021.5010
Abstract
Rezumat
Carcinomul mamar intrachistic reprezintă o formă rară de neoplazie mamară, cu un prognostic excelent şi o rată scăzută de recurenţă. Incidenţa maximă se întâlneşte la femeile în postmenopauză. Evoluţia naturală şi conduita exactă nu sunt încă pe deplin stabilite. Simptomele sunt nespecifice şi explică prezentarea tardivă în vederea evaluării medicale. Uneori, chiar şi investigaţiile precum mamografia, ecografia mamară sau citologia pot subdiagnostica carcinomul mamar intrachistic. Chirurgia conservatoare reprezintă cea mai eficientă metodă de tratament, constând în ablaţia tumorii, cu margini de rezecţie negative de minimum 2 cm, cu sau fără biopsia ganglionului-santinelă. Prezentăm două cazuri de carcinom mamar intrachistic, precum şi o revizuire a literaturii de specialitate.
Introduction
Intracystic (encysted) papillary cancer (IPC) is a very rare form of breast neoplasia with a favorable prognosis, that usually occurs in elderly women.
Its natural history and proper management are not yet fully acknowledged. The disease usually appears in women in their sixth to eighth decades of life, only a few cases being reported in women in their 20s or 30s(1,2). However, this disease does not occur exclusively in females; there are studies that report IPC cases in male patients(3). The incidence of IPC is reported to be 1-2% of all breast tumors(4), and its survival rate is close to 100% at 10 years, the disease-free survival rate being almost 91%(5). However, as it occurs in elderly patients with comorbidities, this survival rate at 10 years may not be accurate. Due to the subtle clinical presentation, it can remain undiagnosed for a long period.
A lobulated circumscribed lesion identified on the mammogram should lead to further investigations. When ultrasonography reveals a complex cystic mass with a solid component, showing vascular flow within the solid component of the cystic mass on color Doppler(6), it should raise the suspicion of this rare diagnosis.
Magnetic resonance imaging (MRI) of the breast with contrast enhancement can further lead to the proper diagnosis(7). The IPC can sometimes be misdiagnosed by the cytological methods, as the fluid aspirated from the cystic component of the tumor could be negative for malignancy and lead to a false-negative result. Thus, the biopsy should target the central part of the solid tumor, or excision biopsy is recommended if invasive cancer is suspected(8). Further, we present two rare cases of IPC and a short review of the literature.
Case report
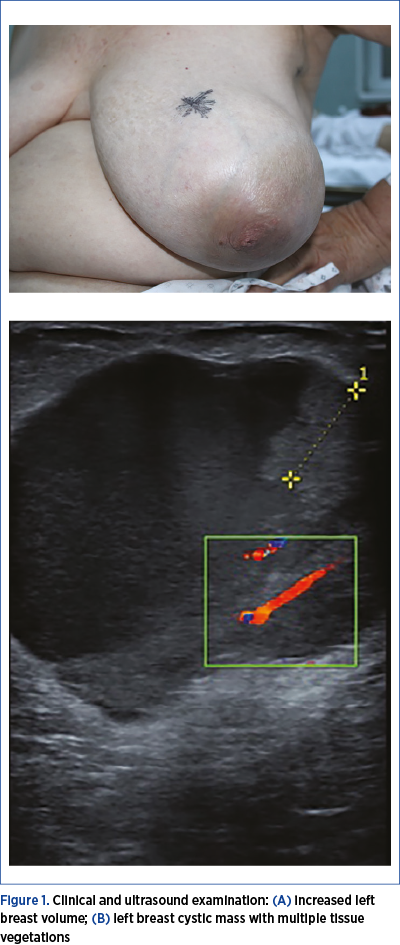
Case 1. A 64-year-old female patient was referred to our clinic for a large lump in her left breast, that increased progressively within the last two years (Figure 1A). The patient had no personal or family history of any pathology, including breast cancer. The patient had a biopsy of the same breast lump two years before, at her first medical examination, with a negative result for malignancy.
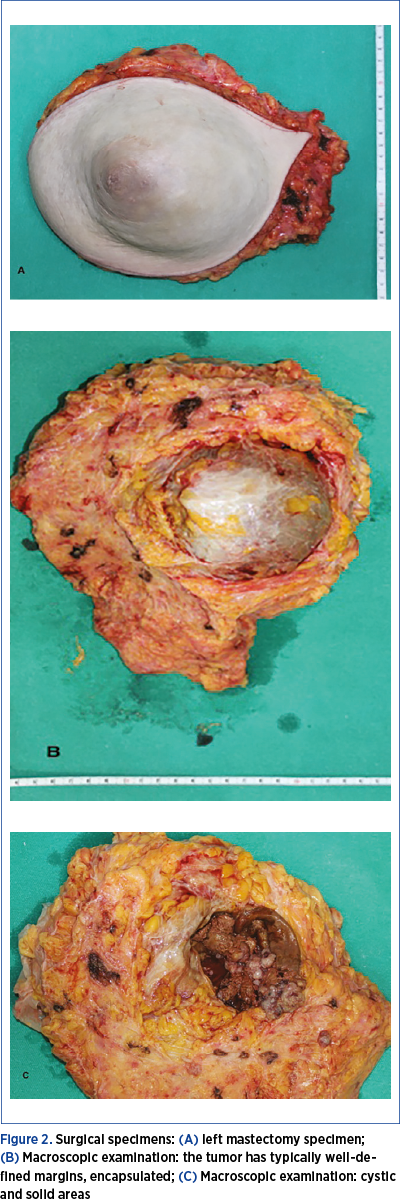
The digital mammography performed in our clinic described an intense circumscribed opacity in the central quadrant of the left breast, measuring 110/90 mm, without spiculate opacities or clustered microcalcifications (BIRADS 2). We further performed a breast ultrasound (Figure 1B), that showed a mixed echogenic tumor (complex cystic mass) with a solid component, with prominent blood supply, measuring 90/90/70 mm; the axillary lymph nodes had no suspicion of metastases. The cytology result was not conclusive for malignancy. Because of the large tumor, involving almost the entire breast, we proceeded to surgery, consisting in mastectomy and sentinel lymph node biopsy (Figure 2 A, B, C). The histopathological examination showed a papillary proliferation with well circumscribed margins, lined by a uniform epithelial proliferation.
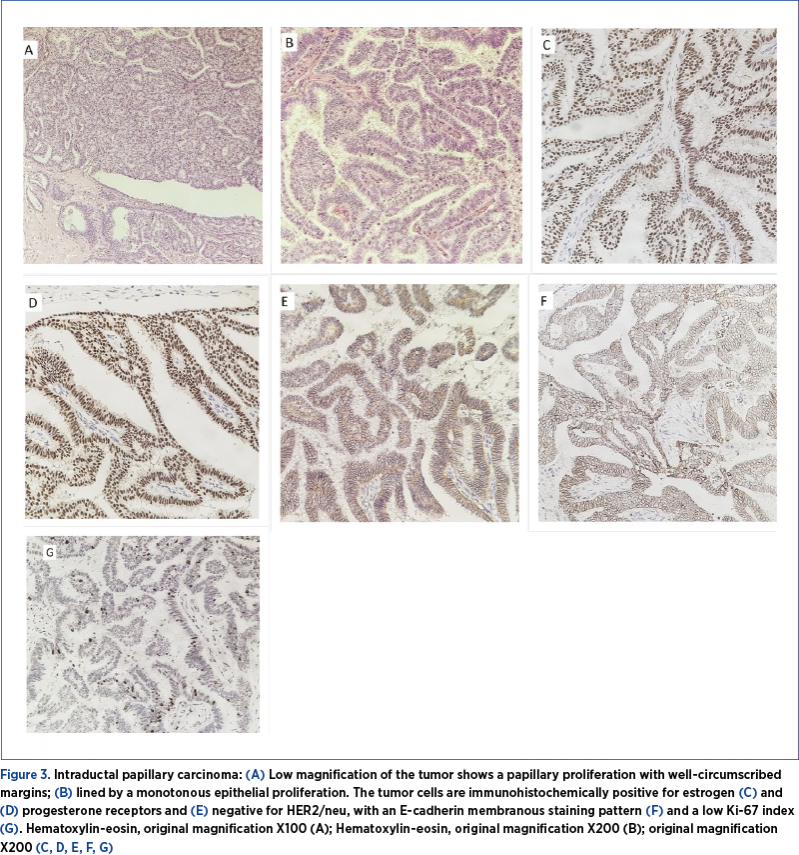
The tumor cells were immunohistochemically positive for estrogen and progesterone receptors and negative for HER2/neu, with an E-cadherin membranous staining pattern and a low Ki-67 index (Figure 3 A, B, C, D, E, F, G), establishing the diagnosis of intraductal papillary carcinoma.
Case 2. A 78-year-old female patient, with no personal or family history of cancer, performed a mammographic examination which described a circumscribed opacity in the central quadrant of the right breast, measuring 40/30 mm, without spiculate opacities or clustered microcalcifications (BIRADS 3).
Breast ultrasound described a mixed echogenic tumor with a solid component, with prominent blood supply, measuring 41/28 mm, and no axillary palpable lymph nodes. The cytology was not conclusive.
Surgery was performed and consisted in wide local excision and sentinel lymph node biopsy. The histopathological examination revealed a papillary proliferation. The tumor cells were immunohistochemically positive for estrogen and progesterone receptors, positive for E-cadherin and with a low Ki-67 index, establishing the diagnosis of intraductal papillary carcinoma.
Both patients underwent adjuvant radiotherapy and tamoxifen therapy, with no signs of local recurrence or distant metastases after 36 months of follow-up.
Discussion
Despite initially classified as a noninvasive form of breast malignancy and a variant subtype of low-grade ductal carcinoma in situ (DCIS), IPC has been lately reported in various studies to occur as an invasive breast cancer in about 40% of cases(9). Due to its favorable prognosis, extensive sampling of the surgical pieces is mandatory in order to avoid misdiagnosing this treatable pathology(9). IPC is frequently reported to be of low or intermediate nuclear grade, without necrosis, although with intense positive estrogen receptor and negative for c-erbB-2(10).
The types of lesions associated with IPC are very important, because they could modify the treatment and change the prognosis. The cases of IPC associated with invasive carcinoma were found to have a nuclear grade 3 and necrosis(11). IPC has been classified into three main subtypes: IPC, IPC with DCIS, and IPC with invasion(9). Clinical data regarding this tumor are limited.
One of the largest studies published in literature, on 917 patients diagnosed with IPC, reported that the relative cumulative survival rate was no different between the group of IPC alone or associated invasive cancer (p=0.18) followed-up at 10 years(3). Regarding the patient’s age, the incidence is higher among older women, the literature describing a single case in a 16-year-old patient(12,13).
The imaging diagnosis of IPC is not specific, with multiple ultrasound features, such as complex mass with solid and cystic areas in IPC associated with DCIS, and irregular tumors with intracystic papillary projections suggestive for invasive IPC(14).
The optimal management is a matter of debate nowadays. The medical strategies include surgical treatment (conservative surgery with or without radiotherapy, or mastectomy)(15), sentinel lymph node biopsy or axillary dissection(16), and hormonal treatment. Chemotherapy is not mandatory, as IPC has low metastasis and vascular invasion rates. However, this should be considered for each patient if invasion has been detected(17). Thus, in order to obtain the best outcome, this challenging pathology should be treated by a multidisciplinary team, including a gynecologist, a radiotherapist and a medical oncologist(18).
The surgical treatment consists in complete tumor excision, which can vary from breast-conserving surgery (wide local excision with 2 mm negative margins(19)), with or without radiotherapy, in order to avoid local recurrence, or mastectomy when breast-conserving surgery is unachievable (large central tumors)(16). During the last decades, the trend changed from radical mastectomy to conservative surgery, as the recurrence rate and cancer-related deaths had no significant difference regardless the type of surgery(3). As there are a few cases of metastatic IPC, axillary surgery (sentinel lymph node biopsy) is not mandatory(10), but still prudent(16). As there is a lack of consensus in the surgical management regarding the sentinel lymph node biopsy (SLNB), in 2017 Wang et al. raised the awareness concerning this issue. They concluded that, even in tumors with a simultaneous invasive component, the lymph nodes are rarely involved and the overall prognosis is very good. Thus, their recommendation was to avoid the routine evaluation of the sentinel lymph node in patients with IPC potentially cured by conservative surgery alone(20). Hassan et al. reported 28 cases in a recent study. Conservative surgery was feasible in half of the patients, with favorable outcome. Axillary evaluation was performed in 84.6% of patients, 53.8% underwent SLNB and 30.8% performed axillary lymph node dissection, with three cases with lymph node metastasis. Furthermore, these authors confirmed an excellent prognosis, but only a meta-analysis of the available literature can lead to the standardization of the treatment of this rare type of breast cancer(12).
Regarding radiotherapy, indications for radiation are not clear in the scientific literature. However, recommendations are of adjuvant radiotherapy for IPC associated with invasion and/or DCIS(21) and in young women (younger than 50 years old) with IPC alone(15,21). Furthermore, a large study performed on 2649 female patients in 2016 concluded that, in order to improve the prognosis, women who underwent conserving surgery for IPC should benefit from radiotherapy(22).
Despite poor evidence-based data to support the use of systemic adjuvant therapy, some clinicians recommend the administration of tamoxifen, as IPC is reported to be almost always hormonal positive and HER-2 negative(21). As many studies sustain that these treatment options used all at once might lead to overtreatment, the therapeutic strategy should be individually assessed. Due to the low risk of metastasis and vascular invasion, chemotherapy is not mandatory(10). However, one case presentation of a giant IPC that firstly received neoadjuvant chemotherapy associated with trastuzumab, without any systemic treatment after surgery (mastectomy with negative sentinel lymph nodes), reported to be in complete remission after 48 months of follow-up(23).
Despite the poor data in the literature regarding the clinical features, preoperative diagnosis, the methods of treatment and the postoperative outcomes of this rare pathology, a recent study performed in China in 2018, on 111 patients diagnosed with IPC, reported some unique characteristics in their population: the proper diagnosis was not established through imaging (easily misinterpreted as benign tumor), nor preoperative core needle biopsies (difficult to perform); axillary lymph node involvement was reported to be rare, as well as distant metastases; the survival rate was excellent (with a better prognosis when compared to Caucasian population and other races); the surgical treatment is the method of choice recommended for IPC(24).
Conclusions
IPC is a rare breast neoplasia and a diagnostic challenge for gynecologists. There are limited data concerning the outcome and many aspects of the optimal management have to be established. There is no standard approach widely accepted regarding the diagnosis and management, and each woman should be individually assessed to recommend the optimal therapeutic strategy.
Bibliografie
-
Baykara M, Coskun U, Demirci U, Yildiz, R, Benekli M, Cakir A, et al. Intracystic papillary carcinoma of the breast: one of the youngest patient in the literature. Med Oncol. 2010;27(4):1427–8.
-
Umanah IN, Okpongette AS. Intracystic papillary carcinoma of the breast in a 21-year old premenopausal Nigerian woman: a case report. Rare Tumors. 2009;1(2):e50.
-
Grabowski J, Salzstein SL, Sadler GR, Blair S. Intracystic papillary carcinoma: a review of 917 cases. Cancer. 2008;113(5):916–20.
-
Rosen PP. Papillary carcinoma. In: Rosen’s Breast Pathology, p. 335–54, 1997.
-
Lefkowitz M, Lefkowitz W, Wargotz ES. Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol. 1994 Aug;25(8):802–9.
-
Liberman L, Feng TL, Susnik B. Case 35: intracystic papillary carcinoma with invasion Radiology. 2001;219(3):781–4.
-
Dogan BE, Whitman GJ, Middleton LP, Phelps M. Intracystic papillary carcinoma of the breast. Am J Roentgenol. 2003;181(1):186-186.
-
Reefy S, Osman H, Chao C, Perry N, Mokbel K. Surgical excision for B3 breast lesions diagnosed by vacuum-assisted core biopsy. Anticancer Res. 2010;30(6):2287–90.
-
Calderaro J, Espie M, Duclos J, Giachetti S, Wehrer D, Sandid W, et al. Breast intracystic papillary carcinoma: an update. Breast J. 2009;15(6):639–44.
-
Reefy SA, Kameshki R, Sada DA, Elewah AA, Awadhi AA, Awadhi KA. Intracystic papillary breast cancer: a clinical update. Ecancermedicalscience. 2013;(7):286.
-
Leal C, Costa I, Fonseca D, Lopes P, Bento MJ, Lopes C. Intracystic (encysted) papillary carcinoma of the breast: a clinical, pathological, and immunohistochemical study. Hum Pathol. 1998;29(10) 1097–104.
-
Hassan Z, Boulos F, Abbas J, El Charif MH, Assi H, Sbaity E. Intracystic papillary carcinoma: clinical presentation, patterns of practice, and oncological outcomes. Breast Cancer Res Treat. 2020;182(2):317-323.
-
Apodaca-Ramos I, Maciel-Roman DA, Tenorio-Torres JA, Kershenovich Gersson J, Moncada-Madrazo M, Said-Lemus FM, et al. Intracystic papillary breast cancer in a 16-year-old patient. Journal of Pediatric and Adolescent Gynecology. 2021;34(2):213-216.
-
Speer ME, Adrada BE, Arribas EM, Hess KR, Middleton LP, Whitman GJ. Imaging of Intracystic Papillary Carcinoma. Curr Probl Diagn Radiol. 2019 Jul-Aug;48(4):348-352.
-
Solorzano CC, Middleton LP, Hunt KK, Mirza N, Meric F, Kuerer HM, et al. Treatment and outcome of patients with intracystic papillary carcinoma of the breast. Am J Surg. 2002;184(4):364–8.
-
Mulligan AM, O’Malley FP. Metastatic potential of encapsulated (intracystic) papillary carcinoma of the breast: a report of 2 cases with axillary lymph node micrometastases. Int J Surg Pathol. 2007;15(2) 143–7.
-
Mugler K, Marshal C, Hardesty L, Finlayson C, Singh M. Intracystic papillary carcinoma of the breast: differential diagnosis and management. Oncology. 2007;21(7):871–6.
-
Gică N, Mustaţă L, Botezatu R, Chirculescu R, Cigaran R, Gică C, et al. Management of Borderline Ovarian Tumors: Series of Case Report and Review of the Literature. Indian J Surg. 2020; https://doi.org/10.1007/s12262-020-02455-w.
-
Harris K, Faliakou E, Exon D, Nasiri N, Sacks NP, Gui GP. Treatment and outcome of intracystic papillary carcinoma of the breast. Br J Surg. 1999;86:1274.
-
Wang Y, Lu S, Graves T, Ouseph MM, Resnick MB, Yakirevich E. Can Sentinel Lymph Node Biopsy Be Spared in Papillary Carcinoma of the Breast? Clin Breast Cancer. 2017 Apr;17(2):127-133.
-
Fayanju OM, Ritter J, Gillanders WE, Eberlein TJ, Dietz JR, Aft R. Therapeutic management of intracystic papillary carcinoma of the breast: the roles of radiation and endocrine therapy. Am J Surg. 2007:194(4) 497–500.
-
Mogal H, Brown DR, Isom S, Griffith K, Howard-McNatt M. Intracystic papillary carcinoma of the breast: A SEER database analysis of implications for therapy. Breast. 2016 Jun;27:87-92.
-
Akladios CY, Roedlich MN, Bretz-Grenier MF, Croce S, Mathelin C. Intracystic papillary carcinoma of the breast: a diagnostic challenge with major clinical impact. Anticancer Res. 2014;34(9):5017-20.
-
Zhang J, Zhang T, Wu N, Zhao X, Wang Q, Jiang Y. Intracystic papillary carcinoma of the breast: Experience of a major Chinese cancer center. Pathol Res Pract. 2018;214(4):579-585.