Cervical primary choriocarcinoma is an extremely rare condition that should be considered in the differential diagnosis of a cervical abnormal bleeding, especially due to intense vasculature, mainly in young women. We present the case of a 31-year-old Caucasian patient, para 2, presenting a cervical well vascularised tumor in the uterine cervix, causing vaginal bleeding that occurred after an evacuated uterine curettage and hemostatically for incomplete abortion. The diagnosis of suspected cervical pregnancy was established based on the imaging aspect: the transvaginal ultrasound showed a parenchymal mass protruding into the cervical canal with intense peripheral vascular network. For differential diagnosis, serum β-hCG dosing was recommended, which had a value of 2110 mUI/ml. The histopathological results correlated with elevated levels of β-hCG suggested the diagnosis of choriocarcinoma. Full interaxial hysterectomy was performed. Choriocarcinoma has a very good prognosis, even in advanced stages, because it is a very chemosensitive tumor.
Coriocarcinom gestaţional al colului uterin – prezentare de caz
Gestational choriocarcinoma of the cervix – a case report
First published: 29 octombrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.3.2019.2616
Abstract
Rezumat
Coriocarcinomul primar al colului uterin este o condiţie extrem de rară, care ar trebui luată în considerare în diagnosticul diferenţial al unei formaţiuni de col uterin cu sângerare abundentă, în special din cauza vascularizaţiei intense, mai ales la femeile tinere. Prezentăm cazul unei paciente în vârstă de 31 de ani, secundipară, care prezintă o formaţiune tumorală bine vascularizată la nivelul colului uterin, cauzatoare de sângerări vaginale debutate în urma unui chiuretaj uterin evacuator şi hemostatic pentru avort spontan incomplet. Diagnosticul de suspiciune de sarcină cervicală a fost stabilit pe baza aspectului imagistic: ecografia transvaginală arată o masă parenchimatoasă care protruzionează în canalul cervical, cu reţea vasculară periferică intensă. Pentru diagnosticul diferenţial se recomandă dozarea β-hCG seric, care a evidenţiat o valoare de 2110 mUI/ml. Rezultatele histopatologice corelate cu nivelurile crescute de β-hCG au sugerat diagnosticul de coriocarcinom. A fost efectuată histerectomie totală interanexială. Coriocarcinomul are un prognostic foarte bun, chiar şi în stadii avansate, deoarece este o tumoare foarte chimiosensibilă.
Introduction
Gestational trophoblastic neoplasia (GTN) includes invasive moles, choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor. These tumors develop almost always with or after a certain form of pregnancy. In the absence of tissues for a definitive histopathological diagnosis, the disease GTN is diagnosed as a result of persistent human chorionic gonadotropin (β-hCG) after evacuation of a molar pregnancy.
Gestational choriocarcinoma is the most common type of trophoblastic neoplasm following a term pregnancy or spontaneous abortion, and only a third of cases occur after a molar pregnancy. Choriocarcinoma consists of reminiscent cells of early cytotrophoblasty and syncytiotrophoblast, but it does not contain vilosities. Choriocarcinomas are usually accompanied by luteal ovarian cysts(1). Gestational choriocarcinoma usually occurs in the uterine cavity and is associated with the coincidence or antecedent of pregnancy. Extrauterine choriocarcinomas are very rare, and most of them are located in the cervix(2).
Case report
A 31-year-old patient with uncertain DUM was hospitalized in our clinic (September 4, 2018) with vaginal bleeding which had started about half a month before, for investigations and specialised treatment. From the patient’s personal history, we found out that two months before (June 21, 2018) she had been admitted to another gynecology clinic with the incomplete abortion clinical diagnosis, the month I/II pregnancy stopped in progress, where she was subject to a hemostatic uterine curettage, evacuator and biopsy with the following histopathological result: tissue fragments representing uterine mucosa with endometrium showing stromal decidualization and advanced progesterone influence glands, abundant inflammatory infiltration of polymorphic character with areas of tissue destruction and numerous fibrous intravascular thrombi.
Following the clinical examination after hospitalization in our clinic, the local exam showed the vulva and vaginal examination of normal appearance, the posterior cervix through which polyposis injuries externalized, the vaginal tact revealed uterus in AVF of quasi-normal dimensions, without tact sensitivity and cervix mobilizing, impalpable annexes, free Douglas.
A transvaginal ultrasound showed a parenchymal mass protruding into the cervical canal with intense peripheral vascular network.
Colposcopic examination: exocol circumscribed by the mammalian ectropion area, at the left epithelium acetoalb epithelium; in the cervical canal polypoid formation with vascular changes at the touch. Biopsy and β-hCG were recommended in dynamics.
On September 5, 2019 (day 1): β-hCG=2110 mUI/mL, and on 10.09.2018 (day 5): β-hCG=2690 mUI/mL.
On September 11, 2018, endocervical curettage (endocervical curettage with sending of extracted material to the histopathological examination, maneuver performed after placing two stitches on the cervicovaginal for hemostatic purposes, placed a Foley probe in the endocolus, minimum metrorrhagia) established the diagnosis of choriocarcinoma endocolar.
Intervention post-day 1 β-hCG=1153 mUI/mL.
The patient was discharged, with good general condition, smooth, without metrorrhagia.
Approximately two weeks after dispensing from our clinic, the patient was hospitalized with minimal metrorrhagia. Day 15 β-hCG was 947 mUI/mL, and the second endocervical curettage was decided in order to send the material to the histopathological examination.
On November 8, 2010, at about two months after the last endocervical curettage, it was intervened surgically and decided to perform the interaxial total hysterectomy.
The histopathological result after total interaxial hysterectomy was as follows: atypical biphasic malignant tumor proliferation with marked cellular pleomorphism of both cytotrophoblast and syncytiotrophoblast, intermediate trophoblast area, the tumor consisting of traveled and trophoblastic mass with cell density, characteristic cell abnormalities, bizarre nuclei, pleomorphic nuclei, multinucleated cells, evident nucleoli, vacuolated cytoplasm, intense mitotic activity, extensive hemorrhagic and tumor necrosis; without corial villities – choriocarcinoma (chorioepitelioma, gestational trophoblastic carcinoma) infiltrates about 2/3 of the endocervix thickness, massive infiltration of the cervical stroma without affecting the uterine istm.
Uterine body with hypertrophied muscle fibers. The endometrium with glands in the proliferative and intermediate phase glands, numerically enhanced, like the simple endometrial glandular hyperplasia, dense corion, without atypia.
Leiomyomatous node with interstitial hialinization, 3 cm in diameter (pT1 Nx Mx).
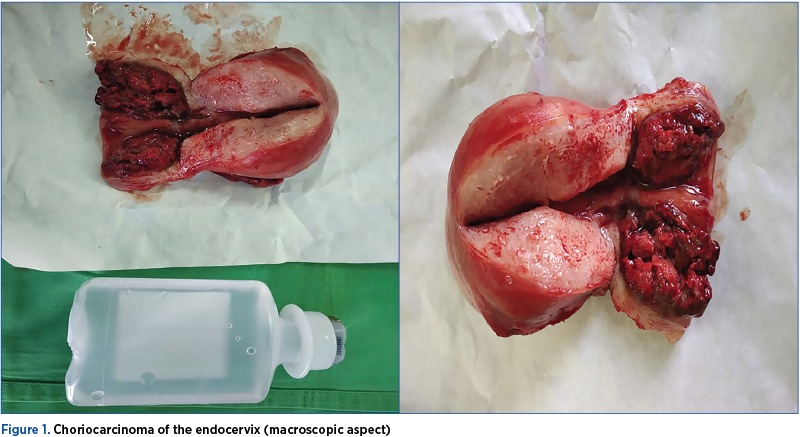
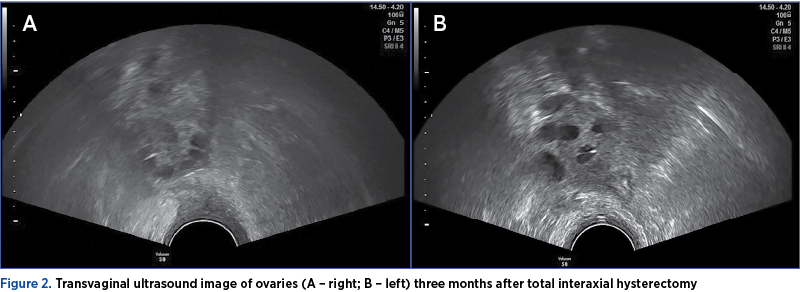
On February 20, 2019, approximately three months after the total interaxial hysterectomy, β-hCG<1.20 mUI/mL (<5.00 mUI/mL – negative).
Discussion
Gestational trophoblastic neoplasia includes invasive moles, choriocarcinoma, trophoblastic placental tumor, and epithelioid trophoblastic tumor. These tumors develop almost always with or after a certain form of pregnancy. Half of the cases occur after a hidatiform molar, a quarter after a spontaneous abortion or a tubal ectopic pregnancy, and another quarter develop after a premature or late delivery. Although these four types of tumors are histologically distinct, they are usually diagnosed only by the persistence of elevated serum levels of β-hCG, as tissue fragments are not always available for histopathology. The criteria for the diagnosis of gestational trophoblastic neoplasia after a molar pregnancy are the following:
-
The serum level of β-hCG in the plateau (±10 percent) at four determinations over a period of three weeks or longer – days 1, 7, 14, and 21.
-
Increase in serum β-hCG>10% at three consecutive weeks or at weekly measurements for two weeks or more – days 1, 7, and 14.
-
The serum level of β-hCG remains detectable for six months or longer.
-
Histological criteria for choriocarcinoma(3).
In women, choriocarcinoma usually arises in the uterine cavity, and is associated with coincident or previous pregnancy(2). Extrauterine choriocarcinoma is a rare entity, with the uterine cervix being the most common site and only a few cases reported in literature to date(2). Several hypotheses have been postulated to explain the pathogenesis of cervical choriocarcinoma. It may develop from cervical metastases from a primary tumor in the corpus that later spontaneously regresses, it is a malignant transformation of a cervical pregnancy, or it is due to transport of chorionic cells from a previous pregnancy that undergo malignant transformations after a dormant period(4). The accurate diagnosis is difficult because of its rarity.
Furthermore, the majority of cases present abnormal vaginal bleeding that could be caused by other more common conditions, including threatened abortion, cervical polyp, cervical pregnancy, or cervical cancer, leading to a potential misdiagnosis(5). Histology with immunohistochemical evaluation remains the mainstay for diagnosis in most cases(5). Considering that choriocarcinoma is a highly chemosensitive tumor with a general good prognosis even in advanced stages, the conservation of reproductive function should be considered, if possible(6).
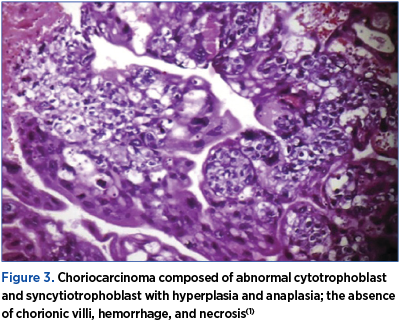
Choriocarcinoma is a malignant disease characterized by abnormal trophoblastic hyperplasia and anaplasia, absence of chorionic villi, hemorrhage and necrosis (Figure 3), with direct invasion into the myometrium and vascular invasion resulting in spread to distant sites, most commonly to the lungs, brain, liver, pelvis, vagina, kidney, intestines, and spleen. Choriocarcinoma has been reported to occur in association with any pregnancy event.
Approximately 25% of cases follow abortion or tubal pregnancy, 25% are associated with term or preterm gestation, and the remaining 50% arise from hydatidiform moles, although only 2-3% of hydatidiform moles progress to choriocarcinoma(1).
These placental tumors are clinically characterized by aggressive invasion in the myometrium and the tendency to metastasis. The most common features of gestational trophoblastic neoplasm are irregular bleeding associated with uterine sub-stimulation. Bleeding may be continuous or intermittent, with sudden and sometimes severe bleeding.
Myometrial perforation caused by trophoblastic proliferation may cause intraperitoneal haemorrhage. In some women, metastases of the inferior genital tract are revealed, while in others there are only distant metastases without signs of a uterine tumor(7).
The recognition of the possibility of gestational trophoblastic neoplasia is the most important element for its diagnosis. Unusual persistent bleeding after any type of pregnancy should promptly lead to the measurement of serum levels of β-hCG and to a diagnosis of uterine curettage. The size of the uterus is evaluated along with careful examination of the inferior genital tract for the detection of metastasis, which usually appears as vivid vascular masses. There is no need for tissue diagnosis, which is why biopsy is not required, because it can cause significant bleeding(8,9).
With the confirmation of the diagnosis, in addition to the initial determination of serum levels of β-hCG and the result of the blood count, investigations are needed to establish the extent of local disease and metastasis, including liver and kidney function tests, transvaginal ultrasound, CT scan or thoracic radiography, CT scan or cranial and abdominal-pelvic MRI.
Less commonly used for the detection of metastasis are positron-emission tomography (PET) scanning and determination of the β-hCG level in the cerebrospinal fluid(10,11).
Gestational trophoblastic neoplasia is clinically staged using the score system of the Federation of the International Federation of Gynecology and Obstetrics (FIGO 2009). This includes a change in the prognostic index score of the World Health Organization (WHO) (1983). Women with WHO scores of 0-6 are included in the low-risk group, while women with a score of ≥7 are included in the high-risk group(12).
It should be stressed again that the diagnosis of trophoblastic neoplasia is usually due to the persistence of elevated serum levels of β-hCG without anatomopathological confirmation. The clinical staging is performed without taking into account the histological findings, even when available(13).
Conclusions
Choriocarcinoma is a malignant variant of trophoblastic gestational disease and may arise from a long-term pregnancy, complicated pregnancy by a molar, ectopic pregnancy or abortion. The incidence of choriocarcinoma after vesicular death is 29-83%. The diagnosis of choriocarcinoma is only histopathological. The present case was a 31-year-old woman with a history of evacuation of a pregnancy outgrowth, two months before. During irregular follow-up, misleading ultrasonographic findings and clinical features delayed the diagnosis of choriocarcinoma until there were external changes in the cervix. We report this case because of its unusual presentation, which has led to a diagnostic dilemma and maladministration. Choriocarcinoma has a very good prognosis, even in advanced stages, because it is a very chemosensitive tumor.
Conflict of interests: The authors declare no conflict of interests
Bibliografie
2. Kairi-Vassilatou E, Papakonstantinou K, Grapsa D, Kondi-Paphiti A, Hasiakos D. Primary gestational choriocarcinoma of the uterine cervix. Report of a case and review of the literature. Int J Gynecol Cancer. 2007 Jul 1;17(4):921–5.
3. Hemschemeyer H, Eastman NJ. Williams Obstetrics. Vol. 51. The American Journal of Nursing. 1951; 32 p. Available from: https://www.jstor.org/stable/3459015?origin=crossref
4. Mangla M, Singla D, Kaur H, Sharma S. Unusual clinical presentations of choriocarcinoma: A systematic review of case reports. Taiwan J Obstet Gynecol. 2017 Feb 1;56(1):1–8.
5. Tse KY, Chan KKL, Tam KF, Ngan HYS. An update on gestational trophoblastic disease. Obstet Gynaecol Reprod Med. 2012; v. 22, n. 1, 7-14.
6. Eysbouts YK, Massuger LFAG, IntHout J, Lok CAR, Sweep FCGJ, Ottevanger PB. The added value of hysterectomy in the management of gestational trophoblastic neoplasia. Gynecol Oncol. 2017; 145(3):536-42.
7. Cagayan MSFS, Gacoba MCC. Chemotherapy regimens used in the treatment of gestational trophoblastic neoplasia at Philippine General Hospital: treatment outcomes and toxicity. J Reprod Med. 2006 Nov; 51(11):907-18.
8. Speroff L, Fritz M. Menopause and the Perimenopausal Transition. In: Clinical gynecologic endocrinology and infertility. 2005; Lippincott Williams and Wilkins.
9. Chen M-J, Yang J-H, Lin M-C, Ho H-N, Yang Y-S. An unusual gestational choriocarcinoma occurring primarily on the surface of a subserous leiomyoma. BJOG An Int J Obstet Gynaecol. 2004 Feb; 111(2):188-90.
10. Roopnarinesingh R, Igoe S, Gillan JE. Choriocarcinoma-presenting as a primary lesion of the cervix. Ir Med J. 2004 May; 97(5):147-8.
11. Chandacham A, Kietpeerakool C, Khunamornpong S, Suprasert P, Srisomboon J, Charoenkwan K, et al. Successfully conservative treatment of large cervical choriocarcinoma with profuse vaginal bleeding. J Med Assoc Thail. 2009 Jan; 92(1):120-3.
12. Clark LH, Staley SA, Barber EL, Wysham WZ, Kim KH, Soper JT. The effect of distance traveled on disease outcomes in gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2016 Aug; 215(2):217.e1-5.
13. Zhao J, Xiang Y, Wan XR, Feng FZ, Cui QC, Yang XY. Molecular Genetic Analyses of Choriocarcinoma. Placenta. 2009 Sep; 30(9):816-20.