A large number of studies have addressed the issue of ovulation, with specific hormones having well-determined roles. Despite the fact that estrogens are known to play a critical role when mature oocytes are set for the process of ovulation, recent studies have disputed the role of the true physiological ovulation trigger, indicating progesterone as the genuine one. The current discussion raises an interesting debate, as progesterone surge may be the real trigger of ovulation. The current mentioned subject needs further investigation in order to ascertain unambiguous conclusions.
Creşterea progesteronului ca declanşator al ovulaţiei
Progesterone surge as the trigger of ovulation
First published: 25 aprilie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.69.2.2021.5003
Abstract
Rezumat
Deşi există numeroase studii care au dezbătut fiziologia procesului de ovulaţie, implicând anumiţi hormoni cu roluri bine determinate, studiile recente au contestat şi rolul principal al estrogenului în ovulaţie, aducând în discuţie un potenţial rol suplimentar şi până acum neglijat al progesteronului. Articolul de faţă îşi propune să treacă în revistă principalele fenomene care stau la baza procesului de ovulaţie, conturând o temă nouă, precum rolul progesteronului în declanşarea ovulaţiei. Acest subiect necesită studii suplimentare pentru a putea trage concluzii solide în ceea ce priveşte rolul progesteronului ca declanşator al ovulaţiei.
Introduction
In the present narrative review, we aimed to revise the main characteristics of ovulation, by describing the leading hormones involved in the process and thus raising a new debate on the capacity of progesterone to trigger ovulation. Literature was searched via PubMed, using keywords such as pregnancy, progesterone, ovulation, menstrual cycle and GnRH, with 18 papers included in the final review.
Ovulation and menstrual cycle
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) carry an important part in controlling the ovulation, a physiologic process that arises when the dominant follicle from the monthly selected ovary is discharged and the oocyte reaches the place of the future fertilization, the salpinges(1).
The menstrual cycle is the consequence of the equilibrium between the hypothalamus, hypophysis and ovaries and is marked by two phases: follicular and luteal(1). A normal menstrual cycle is defined as the period of time from the first day of menstrual bleeding until the first menses day of the subsequent cycle and is characterized by a median period of 28 days, with variations from 25 up to 30 days(1). Ovulation is the process that succeeds the follicular phase and occurs usually on day 14 of the menstrual cycle(1). Energetic cells in the follicle granulosa induce estrogen production, persuading LH surge and follicle discharge(1). Anatomically, the homogametic sex (the female) has two ovaries attached to the uterus through the utero-ovarian ligaments and to the pelvis through the infundibulopelvic ligaments, each ovary accommodating 1-2 million primordial follicles captured in prophase I of meiosis until the pubescence age, when they enter meiosis(1).
Ovulation is a process subordinated to the synergy of distinct hormones, gonadotropin-releasing hormone (GnRH) and gonadotropin hormones(2).
Gonadotropin-releasing hormone
The function of procreation depends on a neuroendocrine basis in which GnRH maintains a fundamental part(2). The hypothalamus includes more than a chiliad of neurons that have the capability to issue gonadotropin-releasing hormone under the GnRH gene expression and, thus, controlling the anterior hypophysis to release LH and FSH which regulate ovulation(2). GnRH responds to various indicators, as they are shown in Table 1(3).
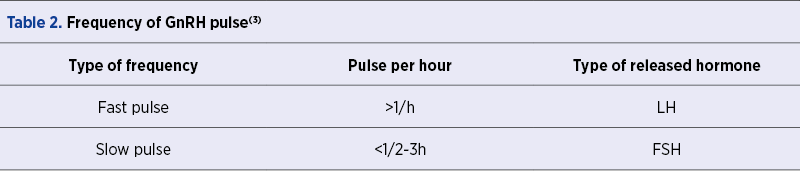
Conjointly with the aforementioned indicators, GnRH release, which occurs in a pulsatile or in a surge form, could also be determined by some factors involved in the natural biorhythm(3). The frequency of GnRH pulsatility influences the type of hormone that is being released, as it is shown in Table 2(3).
Pulsatile releasing of GnRH is influenced by many factors and in women it can cause reproductive afflictions, as presented in Table 3(4). Calorie intake, as suggested in studies that have linked GnRH to functional hypothalamic amenorrhea (FHA), or the lack of adequate calorie intake and excess exercising have the capacity to inhibit GnRH pulsatile-release(4).
Gonadotropin hormones
Glycoproteins that form gonadotropin hormones are composed by an alpha subunit, common in TSH, FSH, LH and CG, and an individual beta subunit, and they are habitually used in fecundity therapy, singular or in distinctive combinations(5).
Gonadotropin-releasing hormone, gonadotropins and their subsequent receptors occur in a form of equilibrium and any pathology that could affect this balance could produce anovulation or amenorrhea(5). The beta subunit plays an important role in the embodiment of each glycoprotein-derived hormone, as it contains two N-glycosylation sites(5).
The role of hCG
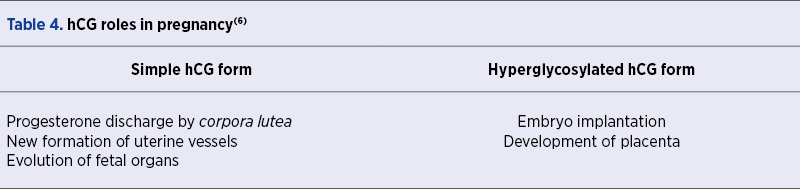
Syncytiotrophoblast contributes to the hCG development during pregnancy, while cytotrophoblast synthesizes a hyperglycosylated isoform, the two hCG forms having different roles in parturiency and egg evolution, as presented in Table 4(6). Besides pregnancy, hCG might play an LH-role during menstrual cycle and, accordingly to Stenman et al., is a sensitive and specific marker for trophoblastic tumors of placental and germ cell origin, the excess production of exclusively beta subunit being a sign of advancing disease and inadequate prognostication(7). Stenman et al. also noted that 45-60% of biliary and pancreatic cancer and 10-30% of other neoplasms are correlated with notable hCG status(7).
FSH and LH
When GnRH is released in a slow pulse (<1/2-3h), FSH is synthesized, a glycoprotein affiliated with the G protein-coupled receptor (GPCR) superfamily, thus regulating follicular growth(8). In a recent review by Pascali et al., it was concluded that b-arrestins, a unique division of proteins, can be mobilized and attached to the gonadotropin receptors, subsequently leading to the potential of pioneering an upgraded therapy for reproduction issues(8,9).
FSH is not exclusively related to the follicular growth, as studies have shown that different levels of FSH are found in osteoclasts and adipocytes(10,11). When GnRH is released in a fast pulse (>1/h), LH is synthesized, a glycoprotein that establishes the appropriate climate for follicular discharge.
Could progesterone surge be the trigger of ovulation?
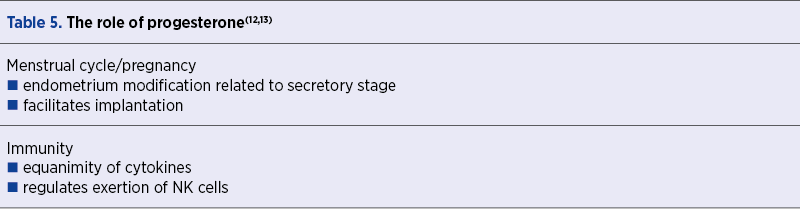
It is undeniable that progesterone plays a fundamental role in the secretory phase and it is crucial to the evolution of menstrual cycle and of a subsequent pregnancy, with plentiful actions, as presented in Table 5(12,13). Besides, during pregnancy, progesterone has the ability to abolish the uterine contractility state through nitric oxide supply(14,15) and to strengthen placental circulation by diminishing pulsatility and resistance index in uterine spiral vessels(16).
In the context of the contemporary science evolution, a new debate emerges: are there additional aspects of progesterone that could actually have a role in triggering ovulation?
In a study conducted by Wang et al. and published in 2019, an important inquiry was brought up: whether there could be a correlation between progesterone levels on the day of hCG or GnRH trigger and on the day of oocyte retrieval in assisted reproductive cycles(17). Any association between late follicular phase progesterone level and fertility is still unclear.
The Wang et al. study was based on 384 women, of which 199 were in the receipt of 5000 IU hCG and 185 under buserelin 0.5 mg for the trigger of ovulation. Although the results indicated an eloquent interrelationship between progesterone levels and ovulation trigger (hCG trigger group p<0.00001 and GnRH trigger group p<0.00001), further studies are needed to be implemented, as during ovarian stimulation, follicles susceptible to elevated levels of FSH will regulate LH receptors, generating high levels of progesterone(17).
Many studies have questioned the role of estradiol in triggering ovulation and, as recent as February 2020, Dozortzev et al. indicated progesterone as a genuine physiological ovulation trigger, especially when progesterone has the capacity to activate gonadotropins which are the key factors involved in ovulation(17). In order to find a solution to this problem, a simple mechanism could be formulated, a mechanism in which progesterone could act as an ovulation trigger in a precise moment at the end of the follicular phase, while remaining as an ovulation inhibitor for the rest of the time of the menstrual cycle or as a part of birth-control medication(18). As a closure, progesterone could act as ovulation trigger, after estradiol surge(18).
Conclusions
Ovulation is a natural process, an evidence of a healthful life. Reproduction in women revolves around ovulation, each ovary containing 1-2 million primordial follicles captured in prophase I until the pubescence age, when they enter meiosis. Although estrogens encompass the prevailing role in the process of ovulation, recent studies have focused on establishing the legitimate part of progesterone.
Further discussions and studies are needed to be implemented in order to reach specific conclusions, especially when reproductive issues continue to play an important role for healthcare providers around the world.
Bibliografie
-
Holesh JE, Bass AN, Lord M. Physiology, Ovulation. [Updated 2020 Aug 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan .
-
Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729-37.
-
Smith JT, et al. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692.
-
Schneider JE, Wade GN. Decreased availability of metabolic fuels induces anestrus in golden hamsters. Wade GN Am J Physiol. 1990 Mar; 258(3 Pt 2): R750-5.
-
Kara E, Dupuy L, Bouillon C, Casteret S, Maurel MC. Modulation of Gonadotropins Activity by Antibodies. Front Endocrinol (Lausanne). 2019;10:15.
-
Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010 Aug 24;8:102.
-
Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem. 2004 Jul;37(7):549-61.
-
De Pascali F, Reiter E. β-arrestins and biased signaling in gonadotropin receptors. Minerva Ginecol. 2018 Oct;70(5):525-538.
-
Kara E, Crépieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol Endocrinol. 2006 Nov;20(11):3014-26.
-
Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006 Apr 21;125(2):247-60.
-
Cui H, Zhao G, Liu R, et al. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. Journal of Lipid Research. 2012 May;53(5):909-917.
-
Carp HJA. Progestogens and pregnancy loss. Climacteric. 2018 Aug;21(4):380-384.
-
Bogdan A, Berta G, Szekeres-Bartho J. PIBF positive uterine NK cells in the mouse decidua. J Reprod Immunol. 2017 Feb;119:38-43.
-
Wolfson ML, Schander JA, Bariani MV, Correa F, Franchi AM. Progesterone modulates the LPS-induced nitric oxide production by a progesterone-receptor independent mechanism. Eur J Pharmacol. 2015 Dec 15;769:110-6.
-
Arrowsmith S, Kendrick A, Wray S. Drugs acting on the pregnant uterus. Obstet Gynaecol Reprod Med. 2010;20(8):241-247.
-
Czajkowski K, Sienko J, Mogilinski M, Bros M, Szczecina R, Czajkowska A. Uteroplacental circulation in early pregnancy complicated by threatened abortion supplemented with vaginal micronized progesterone or oral dydrogesterone. Fertil Steril. 2007 Mar;87(3):613-8.
-
Friis Wang N, Skouby SO, Humaidan P, Andersen CY. Response to ovulation trigger is correlated to late follicular phase progesterone levels: A hypothesis explaining reduced reproductive outcomes caused by increased late follicular progesterone rise. Human Reproduction. 2019 May;34(5):942–948.
-
Dozortsev D, Pellicer A, Diamond MP. Progesterone is a physiological trigger of ovulatory gonadotropins. Fertil Steril. 2020 May;113(5):923-924.
Articole din ediţiile anterioare
Cât de des recomandaţi suplimentarea cu vitamină D în sarcină? Ce trebuie să ştie obstetricienii despre vitamina D şi sarcină
Vitamina D (calciferolul), măsurată prin 1,25-dihidroxivitamina D în serul matern, este importantă pentru dezvoltarea unităţii fetoplacentare. Celu...
Microangiopatii trombotice (PE/HELLP, PTT, aSHU). Diagnosticul diferenţial: date clinice şi de laborator
Cauzele microangiopatiei trombotice identificate în timpul sarcini sunt variate: specifice sarcinii şi nespecifice. Diferenţierea preeclampsiei de ...
Sarcina şi naşterea în timpul pandemiei de COVID-19. Implicaţii pentru gravidă şi naştere
In late December 2019, a series of pneumonia cases of unknown cause appeared in Wuhan City, Central China, which were reported to the World Health ...
Experienţa noastră în gestionarea sarcinii la adolescenţi
Sarcina la adolescente are un impact negativ major în întreaga lume, din cauza complicaţiilor sale materne, fetale şi sociale. Stabilirea unei moda...