The pathogenic mechanism of preeclampsia involves endothelial dysfunction due to impaired angiogenesis, with an imbalance between pro-angiogenic and anti-angiogenic circulating factors. Angiogenesis occurs under both normal and pathological conditions and has an essential role in embryonic and placental development. One of the most important growth factors with a role in the regulation of angiogenesis is the Vascular Endothelial Growth Factor (VEGF), which exerts its function after binding to three receptors, VEGFR-1 (vascular endothelial growth factor receptor 1; flt-1), VEGFR-2 (vascular endothelial growth factor receptor 2; KDR) and VEGFR-3 (vascular endothelial growth factor receptor 3; flt-4). The binding of VEGF to the soluble form of VEGFR (sVEGFR or sflt-1) has an antiangiogenic effect. Preeclamptic placenta overexpresses sflt-1, which enters the maternal circulation, binds VEGF, and determines hypertension and proteinuria in the later stage of pregnancy. Sflt-1 levels could be used as a routine diagnostic test for preeclampsia, to differentiate preeclampsia from other diseases, and as a prognostic factor for imminent delivery in preeclamptic women. Also, sflt-1 could be a target for drug action in the treatment of preeclampsia.
Factori angiogenici în timpul sarcinii normale şi preeclampsia
Angiogenic factors in normal pregnancy and preeclampsia
First published: 21 iunie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.2.2019.2418
Abstract
Rezumat
Mecanismul patogen al preeclampsiei implică disfuncţia endotelială din cauza angiogenezei deficitare, cu un dezechilibru între factorii circulanţi proangiogenici şi antiangiogenici. Angiogeneza apare atât în condiţii normale, cât şi patologice, având un rol esenţial în dezvoltarea embrionară şi placentară. Unul dintre cei mai importanţi factori de creştere cu rol în reglarea angiogenezei este factorul de creştere a endoteliului vascular (VEGF), care îşi exercită funcţia după legarea la trei receptori, VEGFR-1 (receptorul de tip 1 al factorului de creştere a endoteliului vascular; flt-1), VEGFR-2 (receptorul de tip 2 al factorului de creştere a endoteliului vascular; KDR) şi VEGFR-3 (receptorul de tip 3 al factorului de creştere a endoteliului vascular; flt-4). Legarea VEGF de forma solubilă a VEGFR (sVEGFR sau sflt-1) are efect antiangiogenic. Placenta pacientelor preeclamptice supraexprimă sflt-1, care intră în circulaţia maternă, se leagă de VEGF şi determină spre sfârşitul sarcinii hipertensiune şi proteinurie. Sflt-1 ar putea fi utilizat ca test diagnostic de rutină pentru preeclampsie, pentru diferenţierea preeclampsiei de alte boli, şi ca factor de prognostic pentru naşterea iminentă la femeile preeclamptice. De asemenea, sflt-1 ar putea fi o ţintă pentru acţiunea medicamentelor în tratamentul preeclampsiei.
Introduction
Preeclampsia is a pregnancy condition characterized by maternal hypertension and proteinuria developing after the 20th week of gestation, with an impact on both the mother and the fetus(1). As in other obstetric complications, such as gestational hypertension, small for gestational age (SGA) and spontaneous preterm birth, preeclampsia occurs in the presence of the placenta and remits when the placenta is delivered, so the only solution is removal of the placenta and premature birth.
In the mother, preeclampsia can determine liver dysfunction, eclampsia, disseminated intravascular coagulation, renal failure, visual modifications or stroke, while in the fetus, intrauterine growth restriction and premature birth may occur. Also, women with preeclampsia have a higher risk to develop chronic hypertension and cardiovascular diseases later in life(2).
In this review, we will discuss the role of angiogenic VEGF and VEGFR factors in normal pregnancy, as well as the pathogenic role of angiogenic VEGF and sflt-1 in early diagnosis and therapies for preeclampsia.
Normal pregnancy versus preeclampsia
In normal pregnancy, during the early formation of the placenta, there is an invasion of extravillous cytotrophoblasts into the spiral arteries, which determines uterine artery remodeling(3). Pseudovasculogenesis occurs with down-regulation of the expression of adhesion molecules characteristic of epithelial cells and up-regulation of the expression of adhesion molecules characteristic of endothelial cells(4).
The pathogenic mechanism of preeclampsia involves incomplete invasion of cytotrophoblasts into the spiral arteries, placental ischemia, hypoxia and endothelial dysfunction. These changes occur in the first trimester of pregnancy, when preeclampsia is asymptomatic. The placenta in pregnancies complicated by preeclampsia has a reduction in the number of chorionic villi and their vasculature(3).
The pathogenesis of preeclampsia is incompletely understood, even though there are many possible risk factors such as hypertension, thrombophilia, oxidative stress or inflammation. One of the suggested factors is endothelial dysfunction caused by impaired angiogenesis, with an imbalance between pro-angiogenic and anti-angiogenic circulating factors(5).
Angiogenesis, VEGF family and its receptors
Angiogenesis is a process of new blood vessel formation that occurs under both normal and pathological conditions. This process can utilize endothelial progenitor cells, usually derived from the bone marrow, or the preexisting vasculature. In this latter case, endothelial cell activation and protease secretion are required(6).
Under normal conditions, angiogenesis has an essential role in embryonic development and normal tissue growth, the female reproductive cycle including placental development.
Under pathological conditions, enhanced angiogenesis promotes tumor growth, metastasis or other diseases such as psoriasis, rheumatoid arthritis or diabetic retinopathy(6). Impaired angiogenesis also occurs in pregnancy complications, such as preeclampsia.
One of the most important growth factors for the endothelium, with a role in the regulation of angiogenesis, is the Vascular Endothelial Growth Factor (VEGF), also termed Vascular Permeability Factor (VPF)(7,8).
Vascular Endothelial Growth Factor (VEGFs), first described by Senger et al. (1983), represents a family of heparin-binding glycoproteins, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F and Placental Growth Factor (PIGF) obtained by alternative splicing and proteolysis. VEGF is expressed by skeletal and cardiac muscle, macrophages, hepatocytes, platelets, neurons or endothelial cells, as well as by tumor cells(8,9). It is mostly expressed by placental syncytiotrophoblast cells and invasive chorionic trophoblast cells during pregnancy; thus, VEGFs have some physiological role in both fetuses and adults, such as embryonic vasculogenesis, migration, survival, blood vessel growth, and cyclic angiogenesis in the female reproductive system(10,11). VEGF-A mediates increased vascular permeability, VEGF-B is a growth factor, VEGF-C is active in angiogenesis, VEGF-E is found in viruses, and VEGF-F is present in snake venom(7,10,12).
VEGF-A is the initially and the most important discovered VEGF, a homodimer that can exist in different isoforms, which binds to two receptors, VEGFR-1 and VEGFR-2, expressed almost exclusively on endothelial cells. The most important are VEGF110, VEGF121, VEGF145, VEGF148, VEGF162, VEGF165, VEGF183, VEGF189, and VEGF206. VEGF165, VEGF189 and VEGF206 are involved in binding to heparin sulfate and presentation to VEGF receptors(13). VEGF-A expression is stimulated by hypoxia(14).
VEGF exerts its function after binding with high affinity to three receptors belonging to a subfamily of receptor tyrosine kinases (RTKs), namely VEGFR-1 (Vascular Endothelial Growth Factor Receptor 1, or fms-like-tyrosine-kinase receptor 1; flt-1), VEGFR-2 (Vascular Endothelial Growth Factor Receptor 2, or kinase insert domain receptor; KDR) and VEGFR-3 (Vascular Endothelial Growth Factor Receptor 3; flt-4). VEGFR-1 (flt-1) is expressed in endothelial cells, osteoblasts, placental trophoblasts, renal mesangial cells, and some hematopoietic stem cells. VEGFR-1 has an extracellular domain (with seven immunoglobulin-like domains), a transmembrane domain, and also an intracellular domain (with tyrosine kinase activity)(8).
Binding of VEGF to the extracellular domain of VEGFR-1 and VEGFR-2 determines vasculogenesis and angiogenesis (cell proliferation, migration and survival, as well as vascular permeability). Binding to VEGFR-2 also induces lymphangiogenesis. Because VEGFR-3 is expressed in the lymphatic endothelium, binding of VEGF only causes lymphangiogenesis(15). VEGF-B and PIGF are also ligands for VEGFR-1. VEGF has a vasodilator action because it stimulates nitric oxide and prostacyclin synthase in endothelial cells(16).
The VEGF family and the role of VEGFs after binding to VEGFR are presented in Figure 1.
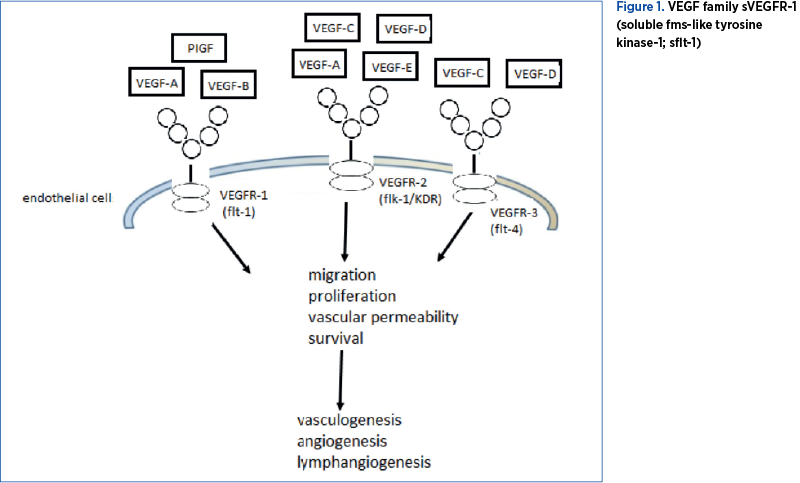
In peripheral blood, there is a soluble, truncated form of VEGF receptor, sVEGFR-1 (soluble fms-like tyrosine kinase-1; sflt-1). It is expressed in the placenta, macrophages and endothelial cells. This truncated sflt-1 form lacks both the transmembrane and intracellular C-terminal domains present in VEGFR-1, but has the site for heparin binding(1). Without the transmembrane domain, sflt-1 can travel free in the blood circulation to other areas in the body(17). Because it contains an extracellular domain identical to that of VEGFR-1, it competes with VEGFR-1 in order to bind with high affinity to VEGF and PIGF. This binding determines reduced serum VEGF and PIGF concentrations, which means that sflt-1 inhibits blood vessel growth in different tissues, kidneys, cornea, and uterus(18-21). The anti-angiogenic effect of sflt-1 is amplified by a second soluble factor, endoglin (sEng), highly expressed in the placenta. The administration of both sflt-1 and sEng in pregnant rats induces hypertension and proteinuria, thrombocytopenia, and fetal growth restriction(5). Also, sflt-1 causes hypertension, because it inhibits the formation of vasodilator endothelial nitric oxide (eNOS).
Pro- and anti-angiogenic factors in normal pregnancy and preeclampsia
Normal angiogenesis is required for oxygen and nutrient supply to the fetus.
In normal pregnancy, natural killer cells in the decidua secrete VEGF. The pro- and anti-angiogenic balance favors VEGF production, which has higher levels in normal pregnancy.
VEGF regulates early placental villous vasculogenesis and branching angiogenesis up to 25 weeks of gestation through its receptors VEGFR-1 and VEGFR-2. Non-branching angiogenesis occurs later until term, and the process is regulated by PIGF. VEGF and VEGFR-2 increase in the first trimester of pregnancy, then their levels decrease during the last months of gestation. On the other hand, PIGF and VEGFR-1 levels increase as pregnancy progresses(22).
On the one hand, PIGF binds to VEGFR-1, allowing VEGF to bind to VEGFR-2; on the other hand, after VEGFR-1 activation, PIGF transphosphorylates VEGFR-2, which amplifies the binding of VEGF to VEGFR-2(3,23).
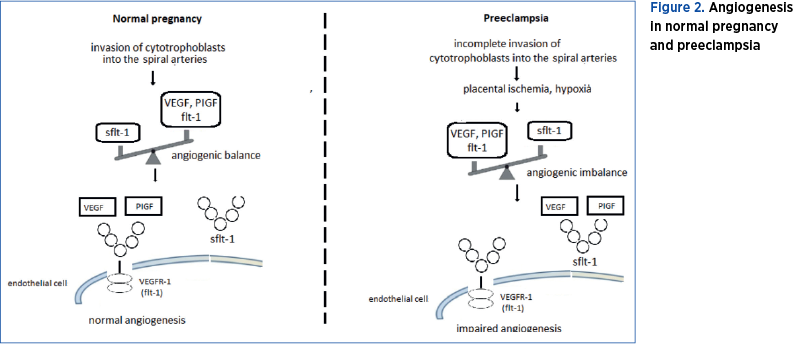
In preeclampsia, endothelial dysfunction is under the control of pro- and anti-angiogenic factors such as VEGF-A, PIGF, VEGFR-1 and sflt-1(1,21). There is an imbalance between pro-angiogenic factors, VEGF and PIGF, and anti-angiogenic factors, sflt-1 and soluble endoglin (sEng), in favor of anti-angiogenic factors. The preeclamptic placenta overexpresses sflt-1, which enters the maternal circulation, binds VEGF, and causes hypertension and proteinuria in the later stage of pregnancy(24).
Sflt-1 binds VEGF and PIGF, decreasing the free VEGF and PIGF levels. The sflt-1/PIGF and sflt-1/VEGF ratio is higher than normal, explaining the decreased nitric oxide production that characterizes the endothelial dysfunction observed in preeclampsia. Because of hypoxia, a condition characteristic of pregnancy, in normal pregnancy sflt-1 is 20-fold increased, but in preeclampsia sflt-1 is about 40-fold increased, which is twice as much as in normal pregnancy, a situation that enhances hypoxia and more sflt-1 is produced(25). Experiments on rats have shown that sflt-1 gene transfer to pregnant rats results in hypertension and proteinuria, symptoms similar to preeclampsia(1). Moreover, Koga et al. (2003) and Tandon et al. (2017) reported that after delivery, the sflt-1 level returned to normal, suggesting the placental origin of sflt-1(26,27).
The interaction of VEGF and PIGF with VEGFR-1 and sflt-1 in normal pregnancy and preeclampsia is presented in Figure 2.
Angiogenic factors as biomarkers for the prediction of preeclampsia
Preeclampsia is diagnosed based on clinical symptoms (hypertension higher than 140/90 mmHg and proteinuria more than 300 mg/24 hours) and uterine artery Doppler velocimetry. There are some laboratory tests that try to predict preeclampsia, but these are not sufficiently sensitive and specific. In order to increase the predictability of preeclampsia before the appearance of clinical symptoms, it is useful to develop reproducible biochemical tests as tools to differentiate preeclampsia from other forms of hypertension in pregnancy(28). Because circulating sflt-1 is produced by the placenta, different studies suggest the implication of sflt-1 in hypertension and proteinuria characteristic of preeclampsia development(21).
The results regarding VEGF and sflt-1 levels during pregnancy are controversial. Some studies showed elevated, other decreased levels of VEGF in preeclampsia and normal pregnant women as compared with non-pregnant women, which is normal because these angiogenic factors have a placental origin. However, most of these studies confirmed decreased levels of VEGF and increased levels of sflt-1 in preeclampsia as compared with normal pregnant women(24,26,29). The studies conducted by Koga et al. (2003), and Levine et al. (2004) indicated that the sflt-1 level increases and VEGF and PIGF levels decrease at least four-five weeks before the onset of preeclampsia, which facilitates earlier diagnosis of preeclampsia(24,26). Knudsen et al. (2012) showed that sflt-1 levels could be used as a routine diagnostic test for preeclampsia. Also, the test could be used in order to differentiate preeclampsia from other diseases, gestational hypertension or gestational thrombocytopenia(30). A higher plasma sflt-1/PIGF ratio is a prognostic factor for imminent delivery in preeclamptic women(31).
The study performed by Muy-Rivera et al. (2005) demonstrated that the risk to develop preeclampsia is 31.6-fold and 56.7-fold increased in pregnant women with higher sflt-1 levels (≥496 pg/ml and 874.4 pg/ml, respectively)(32). There are also differences depending on the severity of preeclampsia, sflt-1 levels being higher and PIGF levels being lower in eclampsia(33). Because sflt-1 has higher levels in primiparous women, the incidence of preeclampsia is higher among first-time-pregnancies.
The observation that PIGF level is decreased in the first trimester of pregnancy in women who later develop preeclampsia and then continues to decrease as sflt-1 concentration increases suggests that these angiogenic factors could be a biochemical test for the prediction of preeclampsia. Moreover, urinary PIGF could be a predictive marker for preeclampsia. The study carried out by Vaisbuch et al. (2011) showed that preeclamptic women with normal sflt-1 and PIGF had no other maternal and fetal symptoms. This result suggests unnecessary intervention for preeclamptic women with a normal angiogenic profile(34).
Even though there are promising results, these tests are not used as routine tests for the prediction of preeclampsia or associated maternal and fetal complications.
Angiogenic factors as targets for pharmaceutical medicine
VEGF and its receptors have received attention because of their role in correcting impaired vessel function. Both VEGF and sflt-1 could be targets for drug action in different pathologies such as atherosclerosis, neurodegenerative diseases or cancerous tumors(35). There are studies which suggest that angiogenic factors could be targets for new medical therapies in preeclampsia treatment.
The study performed by Thandani et al. (2011) demonstrates a 30-40% reduction of sflt-1 using dextran sulfate apheresis. Also, the symptoms of preeclampsia, such as hypertension and proteinuria, are ameliorated and the duration of pregnancy is prolonged for two to four weeks, with no maternal or fetal morbidity. Moreover, the number of cures with dextran sulfate increases the duration of pregnancy(36).
The anti-angiogenic effects of sflt-1 could be reversed by exogenous addition of VEGF or PIGF. Mouse models of preeclampsia attempted to use VEGF as a therapeutic target in order to reduce hypertension and also to restore the angiogenic balance(37). The kidney and liver are tissues that highly express VEGF, binding of VEGF to sflt-1 explaining proteinuria and elevated transaminases. Decreasing the PIGF levels has no effect on proteinuria; the supplementation with PIGF in order to bind sflt-1 is one of the medical solutions. The observation was made using animal models. Also, there are two clinical trials which test the utility of statins and provastatin in preeclampsia prevention: “Statins to ameliorate early-onset preeclampsia” (www.controlled-trials.com ISRCTN23410175) and “Provastatin for prevention of preeclampsia” (www.ClinicalTrials.gov NCT01717586)(37).
VEGF toxicity and the possibility that VEGF crosses the placenta, increasing vascular permeability and impairing the fetal heart, suggest that VEGF is not the most favorable option to treat preeclampsia. The administration of PIGF does not have these negative side effects on the embryos, so PIGF is a safe option(38,39).
Conclusions
Further studies are needed to understand the molecular defect in preeclampsia and the role of angiogenic biomarkers for diagnosis, prognosis and treatment of this obstetric condition. The results regarding anti-angiogenic therapies may help obstetricians for efficient management decisions in preeclampsia.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Bellamy L, Casas JP, Hingorani AD et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007; 335:974.
3. Andraweera PH, Dekker GA, Laurence JA et al. Placental expression of VEGF family mRNA in adverse pregnancy outcomes. Placenta. 2012; 33:467e4724.
4. Zhou Y, Fisher SJ, Janatpour M et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion?. The Journal of Clinical Investigation. 1997; 99 (9): 2139–51.
5. Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest. 2013; 123:2775–2777.
6. Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N. Engl. J. Med. 1995; 26:1757.
7. Senger DR, Galli SJ, Dvorak AM et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219(4587):983-985.
8. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18(1):4-25.
9. Sugishita Y, Takahashi T, Shimizu T, et al. Expression of genes encoding vascular endothelial growth factor and its Flk-1 receptor in the chick embryonic heart. J Mol Cell Cardiol. 2000; 32(6):1039-1051.
10. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25(4):581-611.
11. Demir R. Expression of VEGF receptors VEFGR-1 and VEGFR-2, angiopoietin receptors Tie-1 and Tie-2 in chorionic villi tree during early pregnancy. Folia Histochemica et Cytobiologica. 2009; 47(3): 435–445.
12. Suto K, Yamazaki Y, Morita T et al. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005; 280(3):2126-31.
13. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001; 114(Pt 5):853-865.
14. Shweiki D, Itin A, Soffer D et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992; 359(6398):843-845.
15. Keyt BA, Nguyen HV, Berleau LT, et al. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996; 271(10):5638-46.
16. Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996; 27(6):838-44.
17. Thomas CP, Andrews JI, Liu KZ. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J. 2007; 21: 3885–3895.
18. Khalil A, Muttukrishna S, Harrington K et al. Effect of antihypertensive therapy with alpha methyldopa on levels of angiogenic factors in pregnancies with hypertensive disorders. PLOS One. 2008; 3(7): e2766.
19. Luft FC. Soluble fms-like tyrosine kinase-1 and atherosclerosis in chronic kidney disease. Kidney International. 2014; 85 (2):238–40.
20. Hod T, Cerdeira AS, Karumanchi SA. Molecular Mechanisms of Preeclampsia. Cold Spring Harb Perspect Med. 2015; 5:a023473.
21. Maynard SE, Min JY, Merchan J et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of Clinical Investigation. 2003; 111(5):649–58.
22. Kang MC, Park SJ, Kim HJ, et al. Gestational loss and growth restriction by angiogenic defects in placental growth factor transgenic mice. Arterioscler Thromb Vasc Biol. 2014; 34(10): 2276–2282.
23. Demir R, Kayisli UA, Cayli S, et al. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006; 27(6e7):535e9.
24. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England Journal of Medicine. 2004; 350 (7): 672–83.
25. Wikström AK, Larsson A, Eriksson UJ, et al. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstetrics and Gynecology. 2007; 109(6):1368–1374.
26. Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. The Journal of Clinical Endocrinology and Metabolism. 2003; 88(5):2348–51.
27. Tandon V, Hiwale S, Amle D et al. Assessment of Serum Vascular Endothelial Growth Factor Levels in Pregnancy-Induced Hypertension Patients. Hindawi Journal of Pregnancy. 2017; Volume 2017, Article ID 3179670, 5 pages.
28. Malha L, Podymow T, August P. Hypertension in Pregnancy, pp. 361-373, in Hypertension: A Companion to Braunwald’s Heart Disease (Third Edition) by George L. Bakris and Matthew Sorrentino, Elsevier, pg. 520.
29. Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18.
30. Knudsen UB, Kronborg CS, von Dadelszen P, et al. A single rapid point-of-care placental growth factor determination as an aid in the diagnosis of preeclampsia. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health. 2012; 2: 8–15.
31. Verdonk K, Visser W, Russcher H, et al. Differential diagnosis of preeclampsia: Remember the soluble fms-like tyrosine kinase 1/placental growth factor ratio. Hypertension. 2012; 60:884–890.
32. Muy-Rivera M, Vadachkoria S, Woelk GB, et al. Maternal Plasma VEGF, sVEGF-R1, and PlGF Concentrations in Preeclamptic and Normotensive Pregnant Zimbabwean Women. Physiol. Res. 2005; 54:611-622.
33. Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006; 12:642–649.
34. Vaisbuch E, Whitty JE, Hassan SS, et al. Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am J Obstet Gynecol. 2011; 204:152. e1–152.e9.
35. Brockington A, Lewis C, Wharton S, et al. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004; 30(5):427-46.
36. Thadhani R, Kisner T, Hagmann H, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011; 124(8):940–50.
37. Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012; 206:58 e51–e58.
38. Vuckovic M, Ponting J, Terman B, et al. Expression of the vascular endothelial growth factor receptor, KDR, in human placenta. J Anat. 1996; 188(Pt. 2):361–366.
39. Mátyás M, et al. Particularities of oxidative stress at newborns in Novel Prospects in Oxidative and Nitrosative Stress (edited by Pinar Atukeren), Intechopen Limited, London, 2018; pg. 93-108, http://dx.doi.org/10.5772/intech
Articole din ediţiile anterioare
Microangiopatii trombotice (PE/HELLP, PTT, aSHU). Diagnosticul diferenţial: date clinice şi de laborator
Cauzele microangiopatiei trombotice identificate în timpul sarcini sunt variate: specifice sarcinii şi nespecifice. Diferenţierea preeclampsiei de ...
O analiză a factorilor de risc pentru preeclampsie
Preeclampsia este o tulburare sistemică a sarcinii caracterizată prin diverse manifestări ale disfuncţiei organelor, asociată cu diverse afecţiuni ...
Placenta succenturiată – provocări în diagnosticul şi managementul prenatal. Raport de caz
Anomaliile placentei au fost asociate cu o incidenţă mai mare a morbidităţii şi mortalităţii materne şi fetale. Placenta cu lob placentar accesor e...
Valoarea predictivă a indicilor Doppler ai arterei uterine la 11-14 săptămâni pentru complicaţiile hipertensive ale sarcinii
Introducere. Complicaţiile hipertensive ale sarcinii pot duce adesea la situaţii grave, chiar cu potenţial letal pentru mamă şi făt. Ecografia Dopp...