Bronchopulmonary dysplasia (BPD) is a common disease following the respiratory distress syndrome in premature infants. The disease is particularly frequent in premature babies with a small gestational age, under 28 weeks. The pathogenesis of BPD is complex, its main mechanism being the impairment of the alveolization and vascularization of the lungs. The etiological factors involved in the appearance of the bronchopulmonary dysplasia are: oxygen therapy, mechanical ventilation, neonatal asphyxia, neonatal sepsis, deficient nutritional status. The prenatal factors playing a role in the pathogenesis of the disease are chorioamnionitis, premature rupture of membranes, and intrauterine growth restriction. The main trigger in the pathogenesis of the disease is inflammation acting on the fetal lung during the intrauterine period. This paper presents the case of a patient who developed severe BPD, while the respiratory distress was moderate, and he did not require surfactant therapy or ventilator support. The mother had premature rupture of membranes of over 14 days and chorioamnionitis confirmed by the histopathological examination.
Formă severă de displazie bronhopulmonară – prezentare de caz
Severe form of bronchopulmonary dysplasia – case report
First published: 29 octombrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.3.2019.2617
Abstract
Rezumat
Displazia bronhopulmonară (DBP) este o afecţiune care apare în continuarea detresei respiratorii a prematurului. Boala afectează în special prematurii cu vârsta gestaţională mică, sub 28 de săptămâni. Patogeneza bolii este complexă, mecanismul principal fiind întreruperea procesului de alveolizare şi vascularizaţie de la nivel pulmonar. Factorii etiologici implicaţi în producerea DBP postnatale sunt: oxigenoterapia, ventilaţia mecanică, asfixia neonatală, sepsisul neonatal, statusul nutriţional deficitar. Factorii antenatali cu rol în patogenia bolii sunt coriomniotita, ruptura prematură de membrane şi restricţia de creştere intrauterină. Triggerul principal în patogeneza bolii este inflamaţia, care va acţiona asupra plămânului fetal încă din perioada intrauterină. În această lucrare, prezentăm cazul unui pacient care a dezvoltat o formă severă de DBP în condiţiile în care detresa respiratorie a fost de formă medie şi nu a necesitat terapie cu surfactant sau suport ventilator. Mama a prezentat membrane rupte prematur de peste 14 zile şi corioamniotită confirmată de examenul histopatologic al placentei.
Introduction
Bronchopulmonary dysplasia (BPD), along with retinopathy of prematurity and periventricular leukomalacia are the most frequent chronic complications seen in premature neonates. The etiology of bronchopulmonary dysplasia is complex, multi-factorial, and is based on a small gestational age, as well as on organ immaturity associated with a range of other contributing factors such as: mechanical ventilation, oxygen use, deficient nutritional status, neonatal asphyxia, neonatal sepsis. Some factors are antenatal: chorioamnionitis, premature rupture of membranes, intrauterine growth restriction(1-3).
BPD is a chronic lung disease characteristic to premature infants with a history of respiratory distress syndrome which was treated with prolonged mechanical ventilation and oxygen. The disease is defined as the need of oxygen at 36 weeks corrected gestational age (CGA). The incidence of the disease’s forms described by Northway in 1967 has decreased during the past years as a result of the use of antenatal corticosteroids, the surfactant treatment, and the use of the noninvasive respiratory support from birth instead of the invasive mechanical ventilation(1,2,9). The new form of the disease is characterized by lesions in the canalicular and saccular phase with the impairment of the normal alveolization and vascularization in extremely preterm infants(4). The disease is seen in premature infants who require oxygen therapy and mild respiratory support, as well as in patients who were exposed to prolonged oxygen therapy at a FiO2 of 22-30%(13,16). The incidence of the disease remains high – i.e., 40% in premature infants <1000 g weeks, also determined by the higher survival rate of the extremely preterm infants as a result of the implementation of modern therapies in neonatal intensive care units (NICU)(1,2).
The presence of BPD in the premature patient will lead to an increased hospitalization of the premature infant, to increased perinatal mortality, impaired neurodevelopment and prolonged, sometimes life-long persistent pulmonary dysfunction. There is also a high risk of death during the first year of life.
Prolonged hospitalization, nutrition problems, slow and difficult growth of the infant with BPD lead to a family social crisis and to increased use of emergency services(5).
Case report
We present the case of a male premature newborn, with a gestational age of 28 weeks, weigh: 1100 g, with hospital delivery, GIPI mother, with no relevant family history. No relevant aspects were noted in the personal pathological history. Regarding the evolution of the pregnancy, it is important to note the premature rupture of amniotic membranes over 14 days antepartum and the presence of a maternal inflammatory syndrome with CRP of 5.14 mg/dl and leukocytes within the normal range. The patient followed an antibiotic treatment with clindamycin and complete corticosteroids therapy.
The neonate was born at 28 weeks of gestation, by caesarean section, in breech presentation, with an Apgar score 4/6. Upon birth, he was ventilated with a T-piece for 4 minutes, FiO2=21%, with spontaneous breathing after two minutes of ventilation.
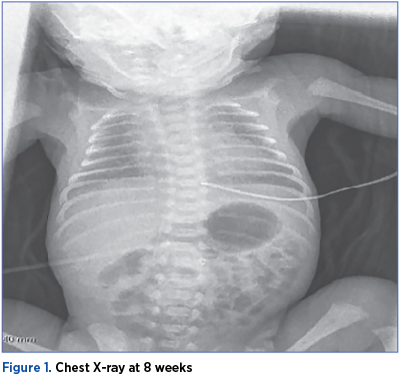
Upon admission to NICU, he had an average general condition, respiratory distress syndrome (RDS) – Silverman score 8, stable hemodynamic. Total parenteral nutrition was initiated, nCPAP respiratory support with FiO2 23% and PEEP=5 cmH2O, caffeine therapy.
The laboratory tests didn’t prove any inflammatory syndrome, and the patient was in normal range metabolically. The immediate evolution was initially favorable after the removal of the respiratory support in the fourth day of life. In the eighth day of life, the respiratory distress syndrome reappeared, leading to the restart of the respiratory support.
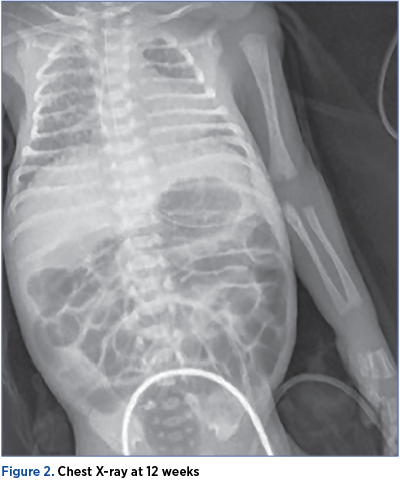
The respiratory evolution was fluctuant, with intermittent removals of the respiratory support, but he remained in need of oxygen throughout the neonatal period. At 36 weeks of age, in need of oxygen therapy and respiratory support, he was diagnosed with bronchopulmonary dysplasia.
Chest X-ray showed bronchopulmonary dysplasia at the age of 8 weeks (36 weeks CGA).
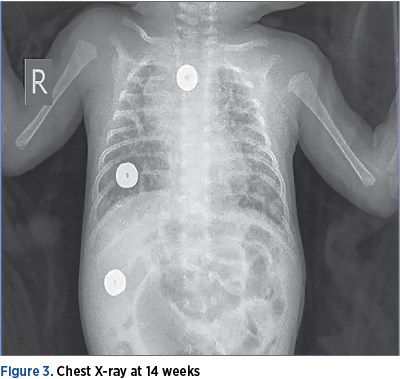
The echocardiogram taken at the age of 2 months (36 weeks CGA) showed the persistence of the small persistent arterial duct with left-to-right shunt, with signs of mild pulmonary hypertension. Based on the clinical context and the paraclinical data, the case was considered a severe BPD.
A complex therapy of parenteral and inhaled corticosteroids, diuretics, pulmonary hypertension treatment (sildenafil) and eternal nutrition (breast milk and milk fortifier) was started, to ensure optimal growth.
The respiratory outcome was not favorable. The patient continued to need oxygen, FiO2 of 23-25%, and NIPPV respiratory support. He had repeated episodes of agitation with desaturation, dyspnea and tachypnea. The inhalation therapy with sympathomimetic beta-2 adrenergic was initiated.
The neurodevelopment was assessed at the age of 50 weeks postconceptional, showing a mixed pyramidal-extrapyramidal frustum image correlated to perinatal asphyxia and prematurity.
Considering this trailing and insufficiently responsive to treatment respiratory symptomatology, other potential causes of the respiratory distress syndrome were taken into consideration. A genetic investigation was carried out for any cystic fibrosis – the analysis of the mutation CFTR c.1521_1523 delCTT - ΔF508 using the Taqman Real-Time PCR Assay, which showed a normal CFTR homozygous genotype.
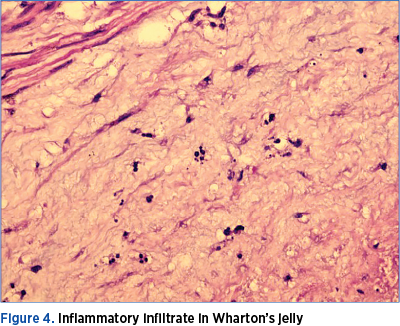
The pathological examination of the placenta revealed abscess areas on the placenta (Figure 4), and an inflammation of the umbilical cord (Figure 5).

Discussion and conclusions
Bronchopulmonary dysplasia is a respiratory disorder seen in premature babies with a small gestational age. Among the perinatal factors, an important part in the pathogenesis of the disease is played by chorioamnionitis, the intrauterine growth restriction, and the premature rupture of membrane. In addition to the prenatal factors, the perinatal factors have a high importance: small gestational age, asphyxia at birth, oxygen therapy, mechanical ventilation and neonatal sepsis(2,3,16).
Pre- and immediately post-natal inflammation is an important trigger in the pathogenesis of BPD. The pathogenesis is complex, the lesions being formed before the disease was diagnosed(11,12).
The factors playing a role in decreasing the incidence of the disease are: antenatal corticosteroids, surfactant therapy, the use of the non-invasive respiratory support, adequate nutrition with sufficient calories to encourage growth, postnatal corticosteroids(13-15).
The particularity of the cases subject to the report consists in the development of a severe form of BPD in a premature neonate who received a complete antenatal corticosteroids therapy, and who did not need postnatal invasive respiratory support, having a small need of oxygen, under 25% in the first days of life. However, the respiratory distress syndrome reappears in the dynamics, trails and requires oxygen therapy at a FiO2 variable between 25% and 35%.
A highlight in this case is the maternal inflammatory syndrome supported by the high C-reactive protein and the premature rupture premature of membrane over 14 days antepartum. Most probably, this is the factor that triggered the pulmonary distress ever since the intrauterine period. The intrauterine inflammation is supported by the pathological examination of the placenta, which revealed multiple abscesses on the placenta and an inflammation of the umbilical cord (Figure 4 and Figure 5).
The surfactant therapy does not influence the evolution of the disease, as the BPD involves a delay in the lung development(11). The main factor in the appearance of the disease is the imperfect angiogenesis. The therapy with vascular development factors would ensure optimal pulmonary vascular development and would prevent the pulmonary impairment induced by hypoxia. In deceased BPD patients, the “old” form of the pathological examination revealed a total absence of normal vascular development(12). Studies carried out proved the beneficial effect of caffeine and vitamin A in decreasing the risk of BPD(6,7). The presented case received caffeine therapy from the first day of life. Vitamin A was not administered in this case.
Prolonged postnatal corticosteroids in the treatment of BPD may have long-term adverse effects for the gastrointestinal system (digestive hemorrhage) and may affect neurocognitive development. There are, however, recent controlled randomized studies (Yeh et al.(8)) which proved the beneficial effect of associating budesonide with surfactant therapy, enabling a swift decrease of the FiO2 under 40% and a decrease in the incidence of BPD in the patients involved in the study(8,9).
At this time, there is no universal acceptance of this therapeutic method, studies been needed in large clinical trials to be able to implement it.
Bronchopulmonary dysplasia remains a complication in premature infants which raises therapy problems and requires prolonged hospitalization of the patient. The severe forms of the disease may impact the patient’s neurological and cognitive development, both as a result of hypoxia and as a result of the prolonged corticosteroid therapy. The severe forms of this disease may appear even in cases of moderate respiratory distress, especially if there are inflammatory triggers, such as chorioamnionitis or neonatal sepsis.
The pathogenesis of the disease is complex and incompletely elucidated, and the therapy is long and difficult. The outcome of this case will be marked by the length of oxygen treatment and the length of other treatments like corticosteroids.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015; 314(10):1039-1051.
3. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016; 375(9):871–8.
4. Jobe AH. The New BPD: An arrest of lung development. Pediatric Research. 1999; 46:641-653.
5. Doyle LW, Ranganathan S, Cheong JLY. Ventilation in preterm infants and lung function at 8 years. N Engl J Med. 2017; 377:1601–1602.
6. Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006; 354(20):2112–21.
7. Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2016; 8. CD000501.
8. Yeh TF, Lin HC, Chang CH, et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008; 121(5):e1310–8.
9. Yeh TF, Chen CM, Wu SY, et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016; 193(1):86–95.
10. Northway Jr WH, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–68.
11. Day CL, Ryan RM. Bronchopulmonary Dysplasia: Old becomes New Again! Pediatric Research. 2017 Jan; 81(1-2):210-213.
12. Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002; 282(4):L811-23.
13. Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014; 105(1):55–63.
14. Carlo WA, McDonald SA, Fanaroff AA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011; 306(21):2348–58.
15. Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: current evidence. Arch Dis Child Fetal Neonat Ed. 2018; 103:F285–F291.
16. Pryhuber GS, Maitre NL, Ballard RA, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015; 15-37.
Articole din ediţiile anterioare
Complicaţii clinice şi ecografice în hemoragia peri-/intraventriculară la nou-născuţii prematuri
Autorii analizează factorii de risc, gradele de severitate şi complicaţiile asociate cu hemoragia peri-/intraventriculară şi stabilesc prin int...
Un caz sever de osteopenie de prematuritate
Osteopenia de prematuritate – denumită şi boala metabolică osoasă sau rahitismul prematurului – este o complicaţie tardivă după naşterea prematură,...