Preeclampsia is a pregnancy-specific systemic disorder characterised by various manifestations of organ dysfunction, associated with different medical conditions that have been shown to increase the risk for this condition, including chronic high blood pressure, diabetes, renal diseases, obesity, and hypercoagulable states. Women who had preeclampsia in a previous pregnancy are also at a higher risk to develop preeclampsia during future pregnancies. In spite of all attempts to predict which patients are more likely to develop preeclampsia and eclampsia, no significant prevention method has been identified yet that could ensure better planning and intervention strategies in case of complications.
O analiză a factorilor de risc pentru preeclampsie
An analysis of risk factors for preeclampsia
First published: 29 octombrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.3.2019.2602
Abstract
Rezumat
Preeclampsia este o tulburare sistemică a sarcinii caracterizată prin diverse manifestări ale disfuncţiei organelor, asociată cu diverse afecţiuni medicale care cresc riscul acestei tulburări, inclusiv hipertensiunea arterială cronică, diabetul zaharat, bolile renale, obezitatea şi stările de hipercoagulabilitate. Femeile cu preeclampsie la o sarcină anterioară au, de asemenea, un risc mai mare de a dezvolta preeclampsie la sarcinile ulterioare. În ciuda încercărilor de a anticipa care paciente sunt susceptibile de a dezvolta preeclampsie şi eclampsie, încă nu s-a stabilit o metodă de prevenire adecvată, care să determine optimizarea planurilor şi strategiilor de intervenţie pentru prevenirea complicaţiilor.
Introduction
Preeclampsia (PE) is a common condition during pregnancy which has been known and studied since the 5th century B.C., when Hippocrates noticed that headache, convulsions and drowsiness were warning signs associated with pregnancy(1).
Recent reports published by the World Health Organization (WHO) estimate that preeclampsia is directly responsible for 70,000 maternal deaths annually worldwide(2).
The National Institute for Health and Care Excellence (NICE) recommends that all patients at risk of preeclampsia be administered a prophylactic dose of aspirin starting at 13 weeks of gestation and until 36 weeks(3).
At least 75 randomized controlled trials have shown that prevention methods effectively and safely prevent preeclampsia in women at moderate or high risk of developing it(4,5).
Women at high risk include those with pre-existing hypertension, chronic kidney diseases, insulin-dependent diabetes, and a personal history of previous early-onset preeclampsia(6).
The understanding of the pathophysiology of preeclampsia, even though it is partial, includes the platelet activation and consumption, vasospasm, and prostacyclin deficiency that have a vasodilatory effect and inhibit platelet aggregation(5).
Materials and methodology
The aim of this study was to monitor 106 pregnant women who underwent obstetric examination and showed risk factors for developing PE, alongside a control group with characteristics similar to the study group.
A database was established from the charts created in compliance with the protocol, with the patients’ consent, who were then subject to dynamic monitoring throughout their pregnancy, including serial ultrasound investigations consisting of Doppler examinations of the uterine and foetal middle cerebral and umbilical arteries which were performed for all patients. The parameters under observation were IR, IP and S/D.
Also, the sFlt/PIGF ratio was calculated using the Elecsys immunoassay sFlt-1/PlGF Roche Diagnostics GmbH in pregnant women with a gestational age between 20 and 32 weeks.
The study also sought to determine the association between risk factors cited in the literature and the risk to develop preeclampsia in the women enrolled in the study.
Women at high risk include those who are nulliparous, at an advanced maternal age, with pre-existing hypertension, chronic kidney diseases, insulin-dependent diabetes, and a personal history of previous early-onset preeclampsia(6).
Findings
1. Socio-demographic characteristics
Maternal age
The age of the women included in the study shows a non-gaussian distribution (p=0.002) – Figure 1.
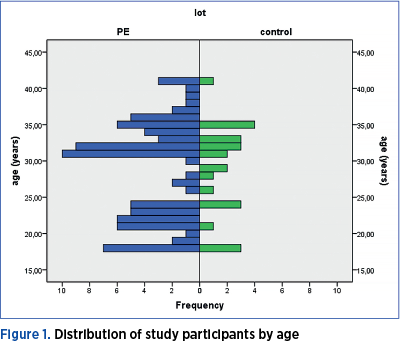
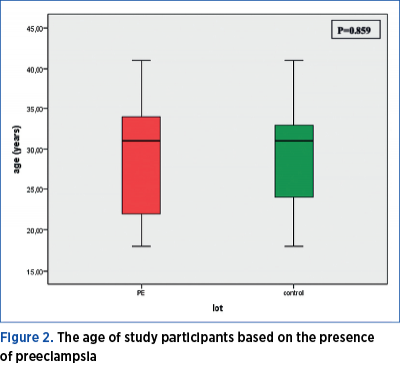
There is no significant age difference between the two groups of women included in the study (Mann Whitney U test = 960.50; p=0.859). Both the pregnant women included in the preeclampsia group, as well as those included in the control group have a median age of 31 years old, the minimum age being 18, and the maximum being 41 years old. 25% of the women in the PE group are younger than 22 years old, and 25% of those included in the control group are younger than 24. The 75th percentile is at the age of 34 years for the study group, and at 33 years old for the control group (Figure 2).
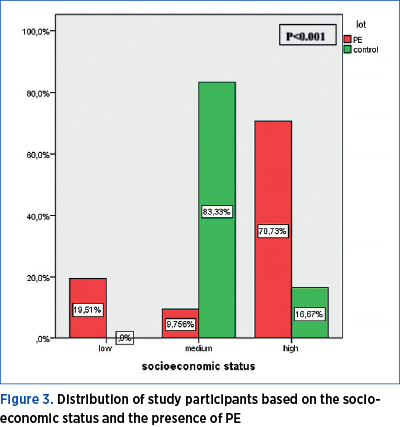
2. Socioeconomic status
A total of 19.51% of women with PE have a low socioeconomic status, 9.76% an average one, and 70.73% a high socioeconomic status. Among the women without PE, 83.33% have an average socioeconomic status, and 16.67% have a high one. Women with high socioeconomic status show a statistically significant higher frequency for PE (p<0.001) – Figure 3.
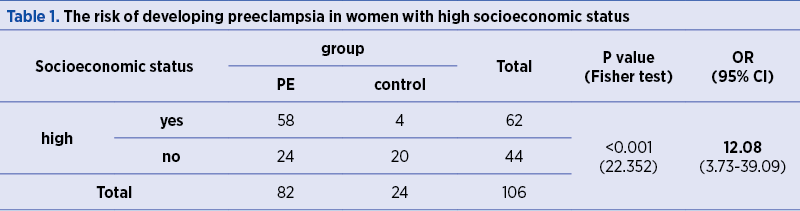
The risk of developing PE is 12.08 higher in women with high socioeconomic status, as compared to women with medium and low socioeconomic status (p<0.001) – Table 1.
The risk of developing PE is significantly lower in women with average socioeconomic status, as compared to women with high and low socioeconomic status (OR=0.02; p<0.001) – Table 2.
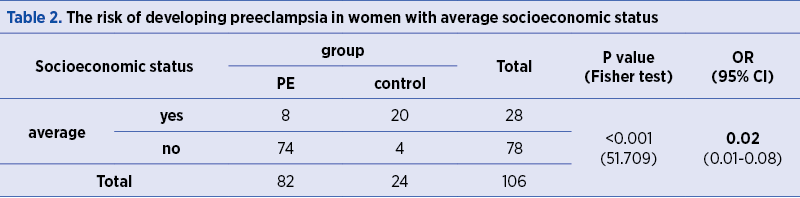
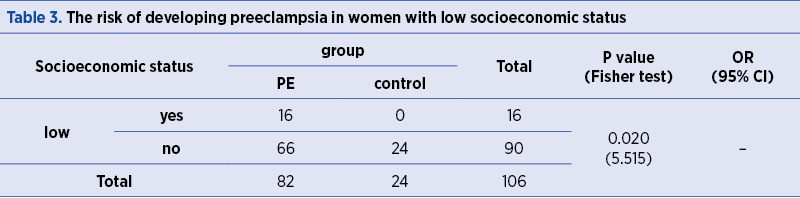
The frequency of PE occurrence is significantly higher (p=0.020) in women with low socioeconomic status (Table 3).
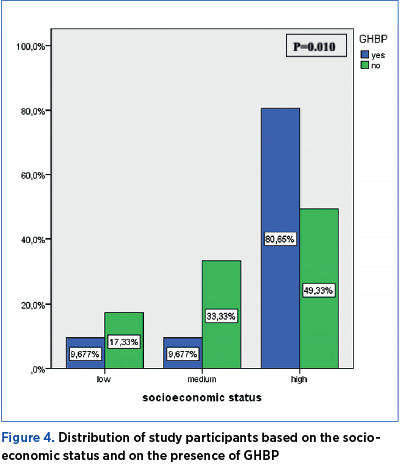
The distribution of study participants based on the socioeconomic status and on the presence of GHBP is significantly different (p=0.010). The prevalence of GHBP is 9.67 in women with low and average socioeconomic status, and 80.65% in women with high socioeconomic status, respectively (Figure 4).
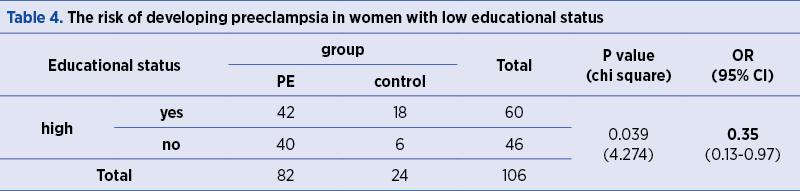
The risk of developing GHBP is 4.28 higher in women with high socioeconomic status, as compared to women with average and low socioeconomic status (p=0.003) – Table 4.
A total of 30.49% of the women participating in the study had completed lower-secondary education, 18.29% had completed upper-secondary education, and 51.22% of participants were women with higher education. In the control group, 25% of the women had a medium educational status, and 75% were women with higher education. The distribution of study participants based on the educational status and on the presence of PE is significantly different (p=0.008) – Figure 5.
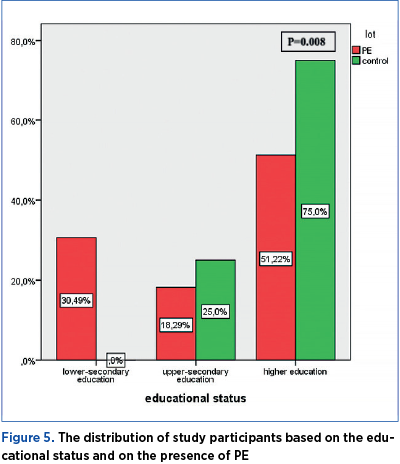
Educational status
The risk of developing PE is 0.35 lower in women with high educational status, as compared to the other participants in the study (OR=0.35; p=0.039) – Table 4.
The risk of developing PE is insignificantly lower in women with a medium educational status, as compared to the other participants in the study (OR=0.67; p=0.468) – Table 5.

The frequency of PE occurrence is significantly higher (p=0.001) in women with low educational status (Table 6).
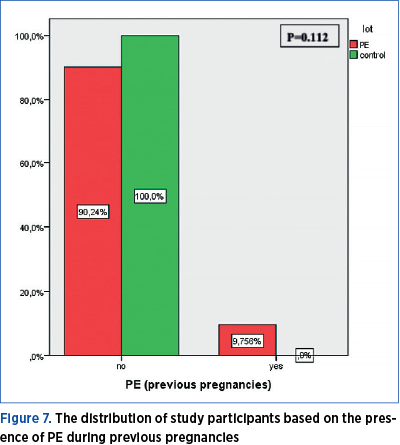
Number of previous births
The frequency of previous births among the participants in the study showed a statistically significant difference (p=0.048) in terms of preeclampsia presence.
The frequency of previous births among the participants in the study shows a statistically significant difference (p=0.048) in terms of preeclampsia presence. 65.85% of the pregnant women with preeclampsia had no previous births, 28.05% had one previous birth, 4.88% had two previous births, and 1.22% had more than two previous births. Among the pregnant women forming the control group, 37.5% had no previous births, 41.67% had one previous birth, 16.67% had two previous births, and 4.17% had a history of more than two previous births (Figure 6).
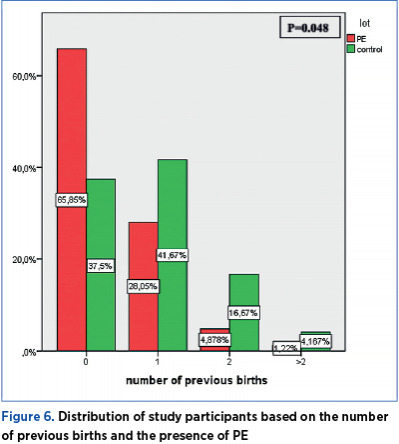
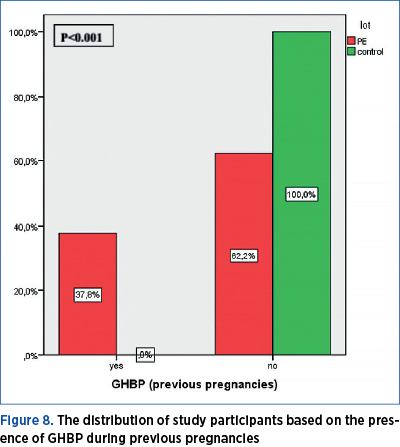
PE during previous pregnancies
Among the 43 pregnant women who had previous births, eight had developed PE during prior pregnancies, and all of them were part of the current PE group. The frequency of PE occurrence during previous pregnancies is significantly different across the two groups under investigation (chi square=5.265; p=0.021) – Table 7.
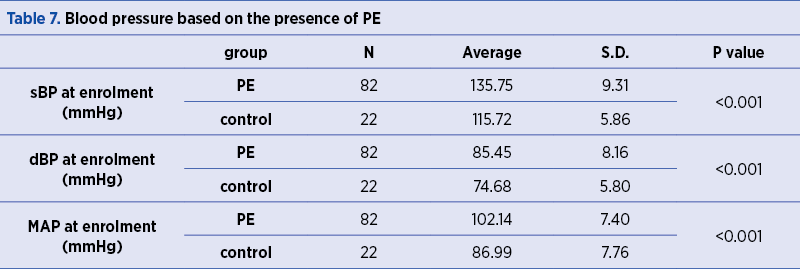
The frequency of GHBP occurrence during previous pregnancies is significantly different across the two groups under investigation (p<0.001). GHBP during previous pregnancy had been reported in 37.8% of women who had PE during the current pregnancy and in none of the women in the control group.
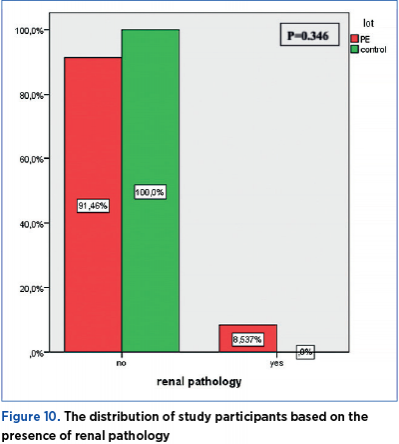
Chronic HBP
A total of 19.51% of the women with preeclampsia have a medical history of chronic HBP, while none of the pregnant women in the control group has a history of HBP. The distribution of study participants based on the presence of chronic HBP shows a significant difference (p=0.020) between the PE group and the control group (Figure 9).
Renal pathology – urinary tract infections
The distribution of study participants based on the presence of renal pathology does not show a statistically significant difference (p=0.346) between the PE group and the control group. 8.54% of women with preeclampsia have a medical history of renal pathology, while none of the pregnant women in the control group has a history of renal pathology (Figure 10).
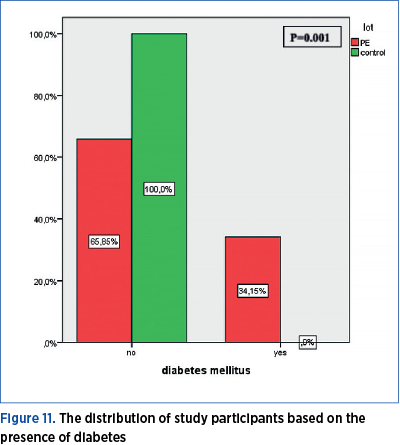
Diabetes
A total of 34.15% of women with preeclampsia have a medical history of diabetes, while none of the pregnant women in the control group has a record of diabetes. The distribution of study participants based on the presence of diabetes shows a statistically significant difference (p=0.001) between the PE group and the control group (Figure 11).
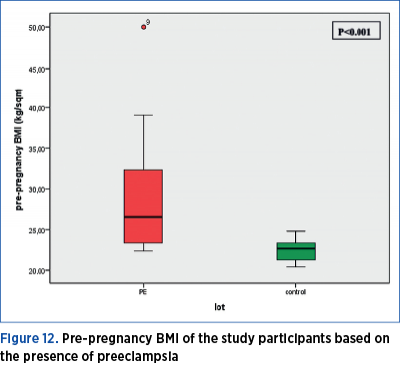
2.1. Weight status before pregnancy
Pre-pregnancy BMI in women with preeclampsia was significantly higher (Mann Whitney U test = 167.50; p<0.001) as compared to the women in the control group. The median value of pre-pregnancy BMI in the preeclampsia group is 26.5, with a minimum value of 22.30, and a maximum of 50; the 25th percentile is at 23.4, and the 75th percentile is at 32.62 kg/sqm. The median value of pre-pregnancy BMI in the control group is 22.6, with a minimum value of 20.40, and a maximum of 24.80; the 25th percentile is at 21.17, and the 75th percentile is at 23.40 kg/sqm (Figure 12).
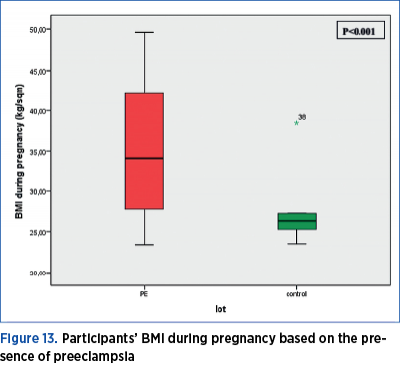
BMI during pregnancy shows a normal distribution (p=0.001). BMI during pregnancy was significantly higher (Mann Whitney U test = 308.00; p<0.001) in women with preeclampsia as compared to the women in the control group. In the preeclampsia group, the median value of the BMI during pregnancy is 34.15, with a minimum value of 23.40 and a maximum of 49.60; the 25th percentile is at 27.67, and the 75th percentile is at 42.10 kg/sqm. In the control group, the median value of the BMI during pregnancy is 26.40, with a minimum value of 23.50 and a maximum of 38.50; the 25th percentile is at 24.92, and the 75th percentile is at 27.30 kg/sqm (Figure 13).
Blood pressure (BP) at enrolment during the second term of pregnancy
In the PE group, the average sBP value at enrolment (135.75±9.31 mmHg) was significantly higher (p<0.001) than the control group (115.72±5.86 mmHg). Moreover, in the PE group, the dBP value at enrolment (85.45±8.16 mmHg) was significantly higher (p<0.001) as compared to the control group (74.68±5.80 mmHg).
The participants in the study who had been diagnosed with PE had a mean arterial pressure (MAP) at enrollment (102.14±7.40 mmHg) significantly higher (p<0.001) than the control group (86.99±7.76 mmHg) – Table 7. Mean arterial pressure is an important component of the different biophysical and biochemical markers used in PE screening. MAP during the second term of pregnancy is a good predictor of a potential risk for preeclampsia.
Discussion
Established risk factors for developing preeclampsia include: primiparity, maternal age above 40 or below 20, chronic hypertension or diabetes, multiparity, a medical history of preeclampsia, as well as BMI above 35, and, in the last decade, the use of assisted reproductive technologies(7,8).
In a document published in 2010, the National Institute for Health and Care Excellence (NICE) proposed a classification of the risk factors for preeclampsia in “moderate risk” and “high risk”, so that they can be used as tools capable of defining the group for which immediate application of prophylactic measures would be indicated(9).
The presence of two moderate-risk factors or a single high-risk factor is considered an indication for prophylactic measures and careful monitoring of the patient. A multicentre study conducted among more than 8,000 nulliparous women with low-risk pregnancy showed a 37% detection rate for preeclampsia using a predictor model composed exclusively of clinical factors(10); obesity and primiparity appeared to be the main demographic predictors of preeclampsia.
Socio-demographic characteristics
In this research, the age of the women included in the study is not significantly different across the two groups (Mann Whitney U test = 960.50; p=0.859), while primiparity constitutes an apparent risk factor; the frequency of births among the participants in the study shows a statistically significant difference (p=0.048) based on the presence of preeclampsia. 65.85% of the pregnant women with preeclampsia had no previous births, 28.05% had one previous birth, 4.88% had two previous births, and 1.22% had more than two previous births.
The risk of developing PE was shown to be 12.08 higher in women with high socioeconomic status, as compared to women with medium and low socioeconomic status (p<0.001). In the study conducted by K. Ramesh(11), there was a considerable difference in terms of illiteracy between the cases with preeclampsia (22%) and the control group (20%).
Personal history
There is compelling evidence suggesting that obesity increases the risk of preeclampsia and cardiovascular diseases(12). In this study, the BMI score during pregnancy was significantly higher (Mann Whitney U test = 308.00; p<0.001) for women with preeclampsia versus women in the control group. These findings are similar to the results reported in the study conducted by J.M. Catov(13) and L.M. Bodnar(14), who concluded that preeclampsia was more prevalent among obese patients.
Diabetes was not seen as a factor associated with preeclampsia; however, the presence of both type 1 and type 2 diabetes is discussed in the literature as a risk factor for developing preeclampsia, with a 15-20 incidence in the cases with type 1 diabetes(15,16) and 10-14% for patients with type 2 diabetes(16,17).
In a study conducted by P. Tubbergen(18), preeclampsia was considered to be a disease specific to first pregnancies. The incidence of preeclampsia decreases during subsequent pregnancies, after a previous normal pregnancy, which leads to the conclusion that multiparity has a beneficial effect. Similar results were obtained in this study; thus, the frequency of births among the study participants shows a statistically significant difference (p=0.048) based on the presence of preeclampsia. 65.85% of the pregnant women with preeclampsia had no previous births, 28.05% had one previous birth, 4.88% had two previous births, and 1.22% had more than two previous births.
A medical history of preeclampsia (HELLP, eclampsia) significantly increases the risk of developing the pathology during future pregnancies, with a major impact on the prognosis and progression of the pregnancy(19,20).
In this study, among the 43 pregnant women who had previous births, eight had developed PE during prior pregnancies, and all of them were part of the current PE group. The frequency of PE occurrence during previous pregnancies is significantly different across the two groups under investigation (chi square = 5.265; p=0.021) – Table 7.
Slight changes in arterial pressure begin to occur in the sixth week of pregnancies, and decrease between week 16 and week 20. Arterial pressure starts to increase again during the peripartum period, and becomes closer to normal values(21).
The risk of preeclampsia is 7.7 times higher in women with chronic high blood pressure, as compared to the general population, according to the results reported in a systemic literature review and meta-analyses in 25 countries(22).
19.51% of women with preeclampsia have a medical history of high blood pressure (HBP), while none of the pregnant women in the control group had a history of HBP. Thus, the distribution of participants in the study based on the presence of chronic HBP shows a significant difference (p=0.020) between the PE group and the control group.
Renal pathology is considered a risk factor for preeclampsia, and vice versa, the presence of preeclampsia could be a risk factor for renal pathology. According to a study conducted by Wang(23), the occurrence of preeclampsia is associated with postpartum microalbuminuria. The distribution of the participants in the current study based on the presence of renal pathology showed no statistically significant difference (p=0.346) between the PE group and the control group. 8.54% of women with preeclampsia have a medical history of renal pathology, while none of the pregnant women in the control group had a history of renal pathology.
Conclusions
Preeclampsia is still one of the leading causes of maternal morbidity. The complexity of its physiopathology is a challenge for future studies. The main goal is to identify the groups at risk by documenting the risk factors and ultimately implementing a significant prevention method able to improve planning and intervention strategies aimed at preventing complications.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005; 365:785-99.
3. National Institute for Health and Care Excellence (NICE) NICE CG 107. Manchester, UK: Institute for Health and Clinical Excellence; 2010. [Accessed February 5, 2015]. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. Available from http://www.nice.org.uk/guidance/cg107/resources/guidance-hypertension-in-pregnancy-pdf.
4. Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007; 2:CD004659.
5. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of preeclampsia among 9364 pregnant women. Lancet. 1994; 343:619-29, 10.1016/S0140-6736(94)92633-6.
6. Kahsay HB, Gashe FE, Ayele WM. Risk factors for hypertensive disorders of pregnancy among mothers in Tigray region, Ethiopia: matched case-control study. BMC Pregnancy Childbirth. 2018; 18: 482.
7. Moussa HN, Alrais MA, Leon MG, Abbas EL, Sibai BM. Obesity epidemic: impact from preconception to postpartum. Future Science OA. 2016; 2(3).
8. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obesity Reviews. 2015; 16(8):621–638.
9. Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. British Medical Journal. 2010; 341, doi: 10.1136/bmj.c2207.
10. Kenny LC, Black MA, Poston L, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: The Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension. 2014; 64(3):644–652.
11. Ramesh K. Socio-Demographic and Other Risk Factors of Preeclampsia at a Tertiary Care Hospital, Karnataka: Case Control Study. J Clin Diagn Res. 2014 Sep; 8(9): JC01–JC04.
12. Roberts JM, Bodnar LM, Patrick TE, Powers RW. The Role of Obesity in Preeclampsia. Pregnancy Hypertens. 2011 Jan 1; 1(1): 6–16.
13. Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe preeclampsia related to pre-existing conditions. International Journal of Epidemiology. 2007; 36:412–9.
14. Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007; 18:234–9.
15. Jensen DM, Damm P, Moelsted-Pedersen L, Ovesen P, Westergaard JG, Moeller M, Beck-Nielsen H. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004 Dec; 27(12):2819-23.
16. Knight KM, Thornburg LL, Pressman EK. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J Reprod Med. 2012 Sep-Oct; 57(9-10):397-404.
17. Groen B, Links TP, van den Berg PP, Hellinga M, Moerman S, Visser GH, Sluiter WJ, Faas MM, Schreuder MC, Visser W, Geelhoed-Duijvestijn PH, Bianchi R, Bartelink AK, de Valk HW. Similar adverse pregnancy outcome in native and nonnative dutch women with pregestational type 2 diabetes: a multicentre retrospective study. ISRN Obstet Gynecol. 2013 Oct 30; 2013:361435.
18. Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, Dekker GA. Change in paternity: a risk factor for preeclampsia in multiparous women? J Reprod Immunol. 1999 Nov; 45(1):81-8.
19. CPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. ECPPA (Estudo Colaborativo para Prevencao Pre-eclampsia com Aspirina) Collaborative Group. Br J Obstet Gynaecol. 1996; 103(1):39-47.
20. Wenstrom KD, Hauth JC, Goldenberg RL, DuBard MB, Lea C. The effect of low-dose aspirin on pregnancies complicated by elevated human chorionic gonadotropin levels. Am J Obstet Gynecol. 1995;173(4):1292-6.
21. Seely EW, Ecker J. Chronic hypertension in pregnancy. N Engl J Med. 2011; 365:439-46.
22. Bramham K. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014; 348, https://doi.org/10.1136/bmj.g2301.
23. Wang IK, Muo CH, Chang YC, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013; 85: 207–213.
Articole din ediţiile anterioare
Microangiopatii trombotice (PE/HELLP, PTT, aSHU). Diagnosticul diferenţial: date clinice şi de laborator
Cauzele microangiopatiei trombotice identificate în timpul sarcini sunt variate: specifice sarcinii şi nespecifice. Diferenţierea preeclampsiei de ...
Infecţiile asociate plăgilor operatorii în pandemia de COVID-19: un studiu comparativ
Pandemia de COVID-19 a impus noi abordări, cu singurul scop de a prioritiza resursele în oferirea, în continuare, de servicii medicale calitative.
Factori angiogenici în timpul sarcinii normale şi preeclampsia
Mecanismul patogen al preeclampsiei implică disfuncţia endotelială din cauza angiogenezei deficitare, cu un dezechilibru între factorii circulanţi ...
Placenta succenturiată – provocări în diagnosticul şi managementul prenatal. Raport de caz
Anomaliile placentei au fost asociate cu o incidenţă mai mare a morbidităţii şi mortalităţii materne şi fetale. Placenta cu lob placentar accesor e...