Background. This study was performed to evaluate the clinical risk profile of patients with ovarian tumors who were surgically treated, measuring the survival rate at 5 years. Method. The medical records of 237 women between ≤30 and ≥60 years old, from the surgery and obstetrics and gynecology clinics of “St. Andrew the Apostle” County Emergency Clinical Hospital, Constanţa, Romania, were analyzed, taking into account the following variables: age, parity, menarche and menopause precocity, gynecological pathology association, serum cancer antigen (CA) 125 marker, and tumor, lymph node and metastasis (TNM) staging and surgical treatment. Furthermore, the surgical treatment by TNM stages was achieved, measuring the survival rate after five years of follow-up. Results. In our study, the highest prevalence of malignant ovarian cancer was seen at ages ≥60 years old (55.69%). Most of the patients with malignant disease were multiparous (81.85%) and the menarche seemed to appear more earlier (i.e., between 10 and 12 years) in about 58.22% of patients. Moreover, from 161 menopausal patients, the higher prevalence was seen at the group between 45 and 55 years old, not being dependent on the earlier appearance. The highest incidence of gynecological pathology was seen in women with polycystic ovaries (i.e., 5.48%). Regarding serum CA125 tumoral marker, higher values were noticed in the majority of patients (89.51%). Most of the patients were diagnosed in stage III TNM (36.58%), followed by stage II (26.58%) and stage IV (20.67%). The highest prevalence of surgical treatment in the first and second stages was represented by total hysterectomy with bilateral anexectomy, omentectomy and peritoneal lavage, and for the third and fourth stages, total hysterectomy, bilateral anexectomy, omentectomy, peritonectomy and lymphadenectomy, with a better survival rate at five years seen in patients under the age of 30 years old. Conclusions. Thus, our study shows the need to create a screening for patients at risk for ovarian cancer which present higher age, multiparity, early menarche, polycystic ovaries association, and higher serum CA126 marker values. The survival rate at five years of folow-up shows a higher incidence of survival in patients under 30 years old, probably due to the earlier stages detected.
Profilul de risc clinic asociat cancerului ovarian
Clinical risk profile associated with ovarian cancer
First published: 18 aprilie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.1.2019.2294
Abstract
Rezumat
Context. Acest studiu a fost efectuat pentru a evalua caracteristicile profilului de risc clinic al pacientelor cu tumori ovariene care au fost tratate chirurgical, măsurând rata de supravieţuire la cinci ani. Metodă. Evidenţele medicale a 237 de femei, între ≤30 şi ≥60 de ani, de la clinicile de chirurgie şi obstetrică şi ginecologie ale Spitalului Clinic Judeţean de Urgenţă „Sf. Apostol Andrei” din Constanţa au fost analizate din punctul de vedere al vârstei, parităţii, menarhei şi precocităţii menopauzei, asocierii patologiei ginecologice, markerului antigenului de cancer (CA) 125 şi stadiului tumoral, ganglionilor limfatici şi metastazei (TNM). Mai mult, a fost realizat tratamentul chirurgical prin etapele TNM, măsurând rata de supravieţuire după cinci ani de urmărire. Rezultate. În studiul nostru, prevalenţa crescută a cancerului ovarian malign a fost observată la vârste ≥60 de ani (55,69%). Majoritatea pacientelor cu boală malignă au fost multipare (81,85%), iar menarha pare să apară mult mai devreme (i.e., între 10 şi 12 ani) la aproximativ 58,22% dintre paciente. Mai mult, din 161 de paciente la menopauză, prevalenţa crescută a fost observată la grupul cuprins între 45 şi 55 de ani, fără a depinde de precocitatea apariţiei. O incidenţă crescută a patologiei ginecologice a fost observată la femeile cu ovare polichistice (5,48%). În ceea ce priveşte valorile serice ale markerului tumoral CA125, valori crescute au fost observate la majoritatea pacientelor (89,51%). Cele mai multe paciente au fost diagnosticate în stadiul III TNM (36,58%), urmat de stadiul II (26,58%) şi de stadiul IV (20,67%). Prevalenţa crescută a tratamentului chirurgical în stadiile I şi II a fost reprezentată de histerectomie totală cu anexectomie bilaterală, omentectomie şi lavaj peritoneal, iar pentru stadiile III şi IV, de histerectomie totală, anexectomie bilaterală, omentectomie, peritonectomie şi limfadenectomie, cu o rată mai mare de supravieţuire la cinci ani la pacientele cu vârsta sub 30 de ani. Concluzii. Riscul apariţiei tumorilor ovariene maligne este asociat mai mult cu vârsta, paritatea, menarha timpurie, asocierea ovarelor polichistice şi bazată pe stadializarea TNM. Rata de supravieţuire la cinci ani ulterior arată o incidenţă mai mare a supravieţuirii la pacientele cu vârsta sub 30 de ani, probabil datorită detecţiei în stadiile incipiente.
Introduction
Being the leading cause of gynecological diseases, ovarian tumors are estimated as the fifth cause of death among women(1). Furthermore, the five-year survival rates of advanced condition after radical surgery still remain below 30%(2,3).
Interestingly, the ovarian tumors are predominantly seen in postmenopausal women, the reproductive age patients representing 20% of the total number of patients(4-6). Many of the published studies are institutional-single center analyses which enrolled only a small number of patients and the majority of reports were not relating to general population(7,8). Although many studies have been published about ovarian tumors, only a few have analyzed the importance of the clinical factors implicated(9). And currently, only a limited number of studies regarding detailed surgical staging have been published, including the survival rate of younger women diagnosed with ovarian tumors(10).
Although for most of the early-detected cases the treatment consisted in total hysterectomy, infracolic omentectomy, peritoneal biopsy and lymph node extraction, maximal cytoreductive surgery remains the basic surgery treatment for advanced ovarian tumors(11-14). Anyway, most of the cases are presented in advanced stages, considering the asymptomatic features of the disease, in which the five-year survival was only 30%(15).
Besides many other tumoral markers involved in diagnosis and prognosis of ovarian cancer, serum cancer antigen (CA)125 is generally used in the differentiation of other pelvic mases(16,17). This marker can be evaluated as a prognostic factor, before the initiation of any treatment(18). However, the implication of serum CA125 levels in ovarian cancer prognostic is more controversial, considering other variabilies such as staging(19).
The present study was undertaken on 237 ovarian cancer patients, in which we proposed to determine the risk associated with age, parity, menarche and menopause precocity, gynecological pathologies, serum CA125 tumoral marker, tumor, lymph node and metastasis (TNM) staging, and surgical treatment associated with improved five-year survival outcome.
Materials and method
The present study was performed on patients with ovarian tumor who were referred to surgery and obstetrics & gynecology clinics of “St. Andrew the Apostle” County Emergency Clinical Hospital, Constanţa, Romania, over a period of five years, between January 2011 till December 2015.
Our study group consisted in 237 patients with malignant ovarian tumors who were selected from a total of 985 ovarian tumors which presented at least one ovarian tumor formation with a 5-mm minimal diameter. All patients underwent surgery as primary treatment. The study was approved by our institution, and the informed consent from each patient was taken. The inclusion criteria were as follows: age between 15 years old and more than 60 years old at the time of the initial diagnosis, all stages of ovarian neoplasms, and receiving only surgical treatment.
We excluded women with a history of tubal sterilization techniques, pelvic radiation therapy either pre- or postoperatively, including pregnant women. We also excluded women with benign and/or borderline tumors.
The tumor stage and histological diagnosis of each case were determined according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO), including TNM staging according to UICC – TNM(20).
There were analized: age, parity, menarche and menopause precocity, gynecological pathology association, serum CA125 when positive if the levels were elevated (≥35 U/mL)(21), TNM staging, surgical treatment by staging and the survival rates after five years. The characteristics were expressed in percentages. Descriptive statistics was used in order to correlate the data.
Results
Distribution by age
Regarding the age of the patients, most malignant ovarian tumors were encountered in the age group over 60 years old, follwed by 30-60-year-old patients, with 39.66% (Table 1).
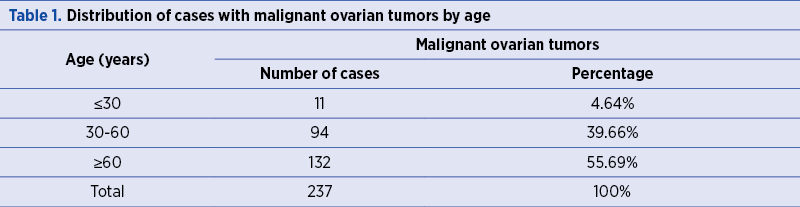
Parity of the patients
Out of the 237 studied women, 194 (81.85%) were multiparous, and 43 (18.14%) were nulliparous (Figure 1).
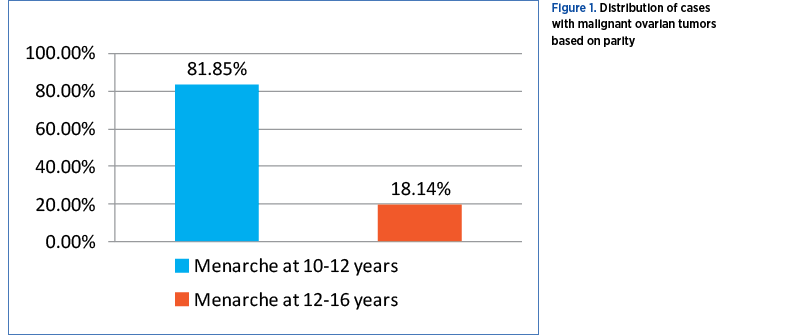
Age of menarche
Malignant tumors occurred in 138 patients (58.22%) having the menarche at 10-12 years old, and in 99 patients (41.77%) with menarhe at 12-16 years old (Figure 2).
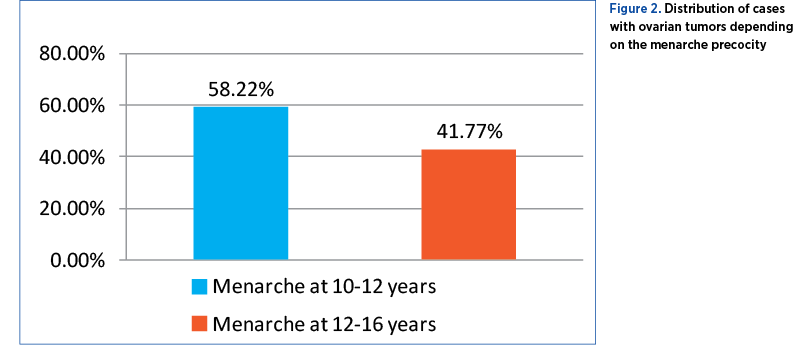
Menopause precocity
Of the 237 cases analyzed, 161 patients were menopausal, with the remaining 76 being in a younger age group. Out of these, 44 (18.56%) had menopause between 35 and 45 years old, and 117 (49.36%) patients had menopause between 45 and 55 years old (Figure 3).
Association of gynecological pathology
Malignant ovarian tumors were associated more with polycystic ovaries, in 13 patients (5.48%), followed by endometriosis in five patients (2.10%), and breast cancer in two patients (0.84%) (Table 2, Figure 4, and Figure 5).
Serum CA125 tumoral marker
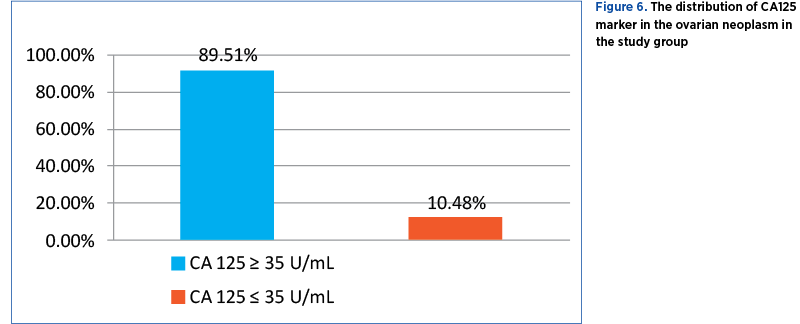
Only 143 cases of malignant tumors were tested for serum CA125 tumor marker. Out of these, 128 (89.51%) patients had elevated values (CA125≥35 U/mL) and 15 (10.48%) patients had normal values (CA125≤35U/mL) (Figure 6).
TNM staging
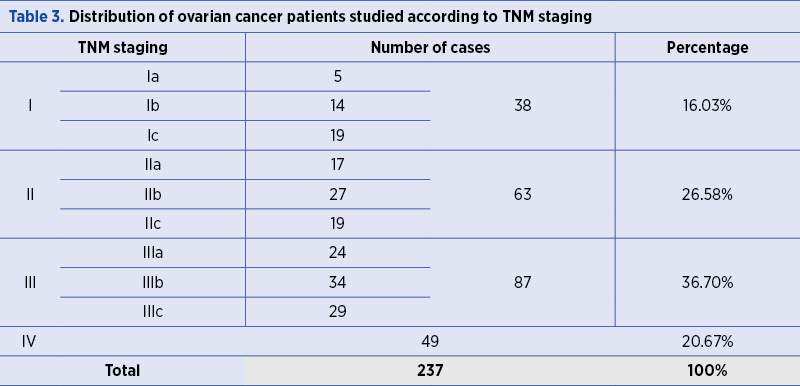
In stage I, there were 38 malignant ovarian tumors (16.03%), of which five were in stage Ia, 14 were in stage Ib, and 19 were in the stage Ic. Stage II represented 26.58% of all ovarian malignancies: 17 cases in stage IIa, 27 cases in stage IIb, and 19 cases in stage IIc. In the third stage, 36.70% of malignant ovarian tumors were classified as follows: 24 were in the stage IIIa, 34 in stage IIIb, and 29 in stage IIIc. In the fourth stage, there were 49 malignant ovarian tumors (20.67%) included (Table 3).
Surgical treatment
The therapeutic strategies have been chosen according to the TNM stage. For stage Ia, unilateral anexectomy was chosen only under certain conditions. Adjuvant chemotherapy was not necessary in all cases. Second-look laparoscopy was practiced at six months per-pelviscopic and was addressed to patients who apparently responded fully to chemotherapy or just to surgical treatment. This allows an assessment of residual risk and consolidation treatment, directing subsequent attitudes. Thus, the following intervention was generally performed for the first and second stages: total hysterectomy with bilateral anexectomy and omentectomy.
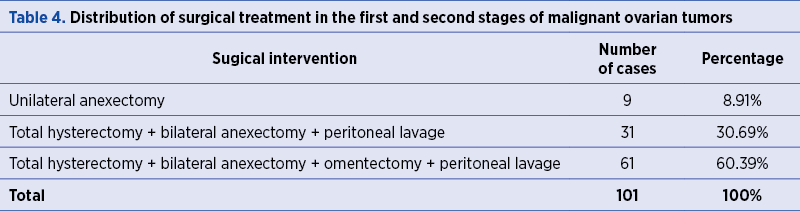
Therefore, malignant ovarian tumors in the first and second stages of development have received the following surgical treatments according to the TNM stage: unilateral anexectomy in 8.91% (9 cases); total hysterectomy with bilateral anexectomy and peritoneal lavage in 30.69% (31 cases); total hysterectomy with bilateral anexectomy, omentectomy and peritoneal lavage in 60.39% (61 cases) – Table 4.
For the third and fourth stages, radical interventions were performed: hysterectomy with bilateral anexectomy with omentectomy, to which the large locoregional and visceral extensions could be added.
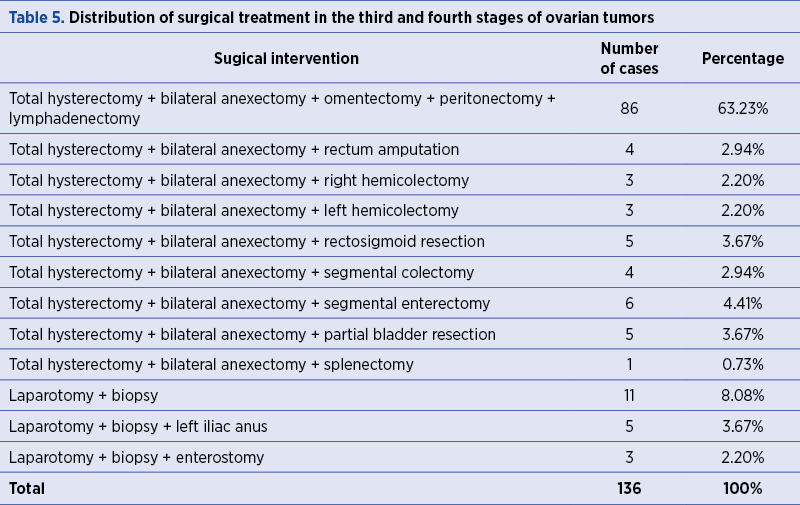
Ovarian cancers in the third and fourth stages were subjected to the following surgical interventions according to the TNM stage: total hysterectomy with bilateral anexectomy, with omentectomy, with peritonectomy and lymphadenectomy in 86 cases (63.23%); total hysterectomy with bilateral anexectomy and rectal amputation in four cases (2.94%); total hysterectomy with bilateral anexectomy and right hemicolectomy in three cases (2.20%); total hysterectomy with bilateral anexectomy and left hemicolectomy in three cases (2.20%) and total hysterectomy with bilateral anexectomy and rectosigmoid resection in five cases (3.67%) – Table 5.
Survival rate after five years depending on age
In the age group under 30 years old, there were 11 women (Ia and Ib stages) with malignant tumors and all survived at five years (100%). The 30-60 age group counted 94 cases with ovarian cancer. Out of these, 50 patients (52.63%; Ic, IIa, IIb and IIc stages) survived at five years. Patients over the age of 60 were 132, of whom only 26 (19.69%; IIIa, IIIb, IIIc, and IV) survived at five years.
Discussion
Many studies involving the clinical risk profile of the malignant tumors are still in debate. Until present, many reports have showed the importance of younger age in the disease prognostic, with better outcome and survival rates(5,22). In this respect, other studies have found opposite results, considering that age was not an independent factor after adjusting the tumor stage(23).
In the present study, we proposed to perform a large population-based study to evaluate the clinical characteristics between younger and older patients with malignant ovarian cancer. Furthermore, we sought to show if younger age is an important factor for improved survival rate, among other features like parity, menarche and menopause, gynecological pathology association, serum CA125 tumoral marker, TNM staging, and surgical treatment.
In our study, the malignant tumors occurred in 58.22% of the patients having the menarche at 10-12 years. In this respect, one study among women population reported lower risk with late age at menarche (i.e., after the age of 18 years old)(24), while another study observed a slightly increased risk with late age at menarche(25). The inconsistent features regarding age at menarche and menopause could show differences and misclassification bias, or differences in study population(26).
Ovarian cancer is predominantly a disease with a median age at diagnosis of 65 years old, most of the women being at menopause. However, approximately 12% of cases occur in women aged less than 45 years old(22). Regarding our study population, it was not surprising to find that the women aged less than 30 were more likely to be in the first stage, and the higher prevalence of malignant ovarian cancer was seen at ages more than 60 years old (55.69%).
Interestingly, another study showed that preoperative CA125 marker is a prognostic feature in advanced malignant ovarian tumors(27). However, the role of serum CA125 remains unknown(28). Serum CA125 represents a glycoprotein expressed in the epithelium lining of body cavities(29), and our study revealed elevated values in majority of patients (5.48%) which were positively correlated with advanced stages. These values could also predict advanced extraovarian disease before surgery(29).
The choice for surgical treatment, especially in early stages of ovarian cancer, usually consist in aspiration of ascites, hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy, bilateral pelvic and para-aortic lymph node sampling(30). Hysterectomy and bilateral salpingo-oophorectomy are more important considering the fact that uterine serosa and endometrium are often sites of occult metastasis(31,32). In the present study, the majority of the patients were in stage III TNM (36.58%), and the higher prevalence of surgical treatment was represented by total hysterectomy with bilateral anexectomy and segmental colectomy/enterectomy.
In our study, the higher survival rate at five years of follow-up was seen in patients under the age of 30 years old, comparing with the rest of the patients. The results are similar with those of the study conducted by Rodriguez and contributors(6) on younger women with ages less than 25 years old and ovarian tumors, which have showed a survival rate of 70% over the five-year period.
Greenlee el al. showed that the first stage of ovarian cancer was associated with a five-year survival rate of 62-85%(33). Moreover, there weren’t seen other negative effects in the patient’s children(34). In the case of patients at fertility ages, they should be informed about surgery consequences and about further fertility preservation therapy(35).
The specific risks in the ovarian cancer in earlier stages before subsequent chemotherapy must be considered and further discussed individually. In the cases when patients undergo chemotherapy, they should wait for about six months in order to eliminate the negative effects on the oocytes(36). Therefore, careful consideration of the ovarian cancer risk profile should better increase the variability in the disease incidence.
Conclusions
In the present study, we sustained the need to create a screening for patients at risk of ovarian cancer which present higher age, multiparity, early menarche, polycystic ovaries association and higher serum CA126 marker values. Furthermore, the prognosis of ovarian cancer showed to be dependent on the clinical profile, in order to better predict the appearance of the disease in early stages.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Nguyen HN, Averette HE, Hoskins W, Sevin BU, Penalver M, Steren A. National survey of ovarian carcinoma. VI. Critical assessment of current International Federation of Gynecology and Obstetrics staging system. Cancer. 1993. 72: 3007-3011.
3. Ries LA, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK. SEER Cancer Statistics Review, 1975-2001, National Cancer Institute: Bethesda, MD. 2004.
4. Smedley H, Sikora K. Age as a prognostic factor in epithelial ovarian carcinoma. Br J Obstet Gynaecol. 1985. 92: 839-842.
5. Swenerton KD, Hislop TG, Spinelli J, LeRiche JC, Yang N, Boyes DA. Ovarian carcinoma: a multivariate analysis of prognostic factors. Obstet Gynecol. 1985. 65: 264-270.
6. Rodriguez M, Nguyen HN, Averette HE, Steren AJ, Penalver MA, Harrison T, Sevin BU. National survey of ovarian carcinoma XII. Epithelial ovarian malignancies in women less than or equal to 25 years of age. Cancer. 1994. 73: 1245-1250.
7. Averette HE, Janicek MF, Menck HR. The National Cancer Data Base Report on Ovarian Cancer. American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1995. 76: 1096-1103.
8. Chan JK, Loizzi V, Lin YG, Osann K, BrewsterWR, DiSaia PJ. Stages III and IV invasive epithelial ovarian carcinoma in younger vs. older women: what prognostic factors are important?. Obstet Gynecol. 2003. 102: 156-161.
9. Yancik R, Ries LG, Yates JW. Ovarian cancer in the elderly: an analysis of Surveillance, Epidemiology, and End Results Program data. Am J Obstet Gynecol. 1986. 154: 639- 647.
10. Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. 2003. 189: 1120-1127.
11. Zanagnolo V, Sartori E, Trussardi E, Pasinetti B, Maggino T. Preservation of ovarian function, reproductive ability and emotional attitudes in patients with malignant ovarian tumors. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2005. 123: 235-243.
12. Jonathan S, Berek, Greenlee RT, Scully RE. Epithelial ovarian cancer. In: Jonathan S, Berek, Neville F, Hacker. Practical Gynecology Oncology, Fourth ed. Williams and Wilkins. 2005. 443-510.
13. Ayhan A, Celik H, Taskiran C, Bozdag G, Aksu T. Oncologic and reproductive outcome after fertility-saving surgery in ovarian cancer. Eur J Gynaecol Oncol. 2003. 24: 223-232.
14. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016. 66: 7-30.
15. Memarzadeh S, Berek JS. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2001. 46: 621-629.
16. Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJ, Soletormos G, Torre GC, et al. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer. 2005. 15: 679e91.
17. Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008. 54: e11e79.
18. Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014. 13: 129.
19. Zorn KK, Tian C, McGuire WP, Hoskins WJ, Markman M, Muggia FM, et al. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: a Gynecologic Oncology Group Study. Cancer. 2009. 115: 1028e35.
20. Union for International Cancer Control (UICC) – TNM Classification of Malignant Tumors, 8th Edition, Edited by James D. Brierly, Mary K. Gospodarowicz and Christian Wittenkind, 2017, John Wiley&Sons LTD, p. 176.
21. Rustin GJS, Nelstrop A, Mcclean P. Use of serum CA125 to define response to first and second line chemotherapy for ovarian cancer – Mount Vernon Hospital, Middlesex. Proceedings of ASCO. 1996. 15: 295.
22. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2012, National cancer institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
23. Massi D, Susini T, Savino L, Boddi V, Amunni G, Colafranceschi M. Epithelial ovarian tumors in the reproductive age group: age is not an independent prognostic factor. Cancer. 1996. 77: 1131-1136.
24. Shu XO, Brinton LA, Gao YT, Yuan JM. Population-based casecontrol study of ovarian cancer in Shanghai. Cancer Res. 1989. 49: 3670-3674.
25. Tzonou A, Day NE, Trichopoulos D, Walker A, Saliaraki M, Papapostolou M, et al. The epidemiology of ovarian cancer in Greece: a case-control study. Eur J Cancer Clin Oncol. 1984. 20: 1045-1052.
26. La Vecchia C. Epidemiology of ovarian cancer: a summary review. Eur J Cancer Prev. 2001. 10: 125-129.
27. Cooper BC, Sood AK, Davis CS, Ritchie JM, Sorosky JI, Anderson B, et al. Preoperative CA 125 levels: an independent prognostic factor for epithelial ovarian cancer. Obstet Gynecol. 2002. 100: 59e64.
28. Koh SC, Huak CY, Lutan D, Marpuang J, Ketut S, Budiana NG, et al. Combined panel of serum human tissue kallikreins and CA-125 for the detection of epithelial ovarian cancer. J Gynecol Oncol. 2012. 23: 175e81.
29. Reiter MJ, Costello JE, Schwope RB, Lisanti CJ, Osswald MB. Review of commonly used serum tumor markers and their relevance for image interpretation. J Comput Assist Tomogr. 2015. 39: 825e34.
30. Benjamin I, Morgan MA, Rubin SC. Occult bilateral involvement in stage I epithelial ovarian cancer. Gynecol Oncol. 1999. 72: 288-291.
31. Morice P, Wicart-Poque F, Rey A, et al. Results of conservative treatment in epithelial ovarian carcinoma. Cancer. 2001. 92: 2412-2418.
32. Park JY, Kim DY, Suh DS, et al. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: oncologic safety and reproductive outcomes. Gynecol Oncol. 2008. 110: 345-353.
33.Greenlee RT, Hill-Harmon MB, Murray T and Thun M. Cancer statistics. CA Cancer J Clin. 2001. 51: 15-36.
34. Zhang R, Sun YC, Zhang GY, Wu LY, Zuo J. Treatment of malignant ovarian germ cell tumors and preservation of fertility. Eur J Gynaecol Oncol. 2012. 33: 489-492.
35. Henes M, Neis F, Kramer B, Walter C, Bucker S, Von Wolff M, Rothmund R, Lawrenz B, Rall K. Possibilities of fertility preservation in young patients with ovarian cancer. Anticancer Research. 2014. 34: 3851-3854.
Articole din ediţiile anterioare
Experienţa noastră în gestionarea sarcinii la adolescenţi
Sarcina la adolescente are un impact negativ major în întreaga lume, din cauza complicaţiilor sale materne, fetale şi sociale. Stabilirea unei moda...
Managementul infecţiei cu SARS-CoV-2 în cazul pacientelor gravide. Cunoştinţele actuale privind COVID-19 în sarcină
SARS-CoV-2 – un nou tip de betacoronavirus ARN – infectează celulele epiteliale de la nivelul căilor respiratorii. Pacienţii cu COVID-19 manifestă ...
Managementul terapeutic al glioblastomului în timpul sarcinii: prezentare de caz subliniind caracteristicile clinice şi radiologice la diagnostic şi după intervenţie
Heterogeneous malignant cerebral tumors include the following types: anaplastic astrocytoma, glioblastoma multiforme type, gliosarcoma and analplas...
Managementul hemoragiei post-partum, în funcţie de cauza de bază
Hemoragia post-partum (HPP) continuă a fi o cauză importantă de deces la nivel mondial. HPP este definită în mod clasic ca o pierdere de sânge de 5...