The screening of cervical cancer is considered to be one of the most efficient prevention programs of malignant diseases. Besides the fact that the introduction of the Pap smear test was associated with the reduction of the incidence and the mortality from cervical cancer, the discovery of the human papillomavirus (HPV) and its carcinogenic potential has started a new era into the screening and prevention of this pathology. The main objective of this study is to evaluate the value of cervical smear measured according to its correspondence between the biopsy result and histopathological conization or hysterectomy exam.
Valoarea frotiului Papanicolau în diagnosticarea displaziilor şi a cancerului de col uterin
The Pap smear test value in dysplasia and cervical cancer diagnosis
First published: 16 aprilie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.69.1.2021.4788
Abstract
Rezumat
Screeningul cancerului de col uterin este considerat unul dintre cele mai eficiente programe de prevenire a bolilor maligne. Introducerea examenului citologic simplu Babeş-Papanicolau a fost asociată atât cu reducerea incidenţei, cât şi cu reducerea ratei de mortalitate prin cancerul cervical, dar descoperirea virusului papilomatozei umane (HPV) şi a potenţialului său cancerigen a introdus o nouă eră în screening şi prevenire. Obiectivul principal al acestui studiu este de a determina valoarea examenului citotumoral cervical, măsurată în funcţie de corespondenţa cu rezultatul biopsiei de etapă şi, respectiv, cu examenul histopatologic al piesei de conizaţie, respectiv histerectomie.
Introduction
Cervical cancer is a worldwide public issue and one of the most preventable malignancies among the relevant human cancers(1). The genesis of this tumor depends essentially on a uterine cervix infection with the human papillomavirus (HPV) that persists for many years or even decades. Cervical cancer might be prevented or at least diagnosed at an early stage, when taking into account that the cervix is an organ easily accessible to clinical evaluation(2).
Based on the epidemiological data for increased risk in cancer development, the HPV genotypes are classified as high risk (hrHPV) and low risk (lrHPV). Among these, HPV16 and HPV18 collectively contribute to about 70% of cervical cancers(3).
Most developed countries have standard cytology-based screening programs to help prevent cervical cancer by detecting premalignant lesions examined on exfoliated cervical cells. Subsequent biopsies can be taken on women with abnormal cytological tests to confirm or deny the presence and severity of cervical intraepithelial neoplasia (CIN) by histology. Cytology-based primary screening programs might be able to prevent up to 80% of cervical cancers in developed countries; however, disease abnormalities have often been missed or misclassified as a result of the test’s relatively low sensitivity and to varying sampling techniques(4,5).
Screening tests have traditionally identified preinvasive or invasive lesions but, on the other hand, cervical cytology may give false positive results which lead to unnecessary interventions or false negative results by undiagnosed preneoplastic or neoplastic diseases. The Papanicolaou stain – worldwide known as Pap test – is the most high-performing method used for cervical screening. The Papanicolaou smear or cervical cytology has been the mainstay of cervical cancer screening for approximately 100 years(6,7).
Materials and method
The current study is a retrospective one, conducted for four years (January 2016 – March 2020), carried out by the First Department of Obstetrics and Gynecology, “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca. The study involved cervical dysplasia cases and incipient forms of cervical cancer diagnosed through cytology – colposcopy and biopsy. As an addition for the study, the clinical observation form was used together with the cytopathological and anatomopathological result records.
The inclusion criteria contained in sexually active and nonpregnant women with no active disease of the cervix and no history of cervical conization, cryotherapy or other invasive cervical cancer treatment, with no history of preinvasive lesions or cervical cancer.
During the four-year study, approximately 2710 Pap smear tests were performed. There were 130 women included, but the date-tracking allowed only 109 women in the study, from whom 4.02% needed surgical procedures.
Based on the anamnestic data and the clinical examination, it was aimed to classify the selected cases depending on age, demographic characteristics, smoking habits, pathological and physiological personal history, cytological result, the diagnosis correspondence between biopsy and cytology, between cytology and histopathological result for conization, respectively hysterectomy.
Conventional Pap smears were taken with a cytobrush from the fornix and the endocervix. Pap smear tests were reported based on Bethesda system 2014.
The common colposcopy steps were made – the cervix examination without any preparation, after applying the 5% acetic acid, the Lugol iodine and collecting tissues samples throughout the colposcopy that have been interpreted by the pathological department.
All subjects with positive PAP smear test underwent colposcopy and biopsy (as a gold standard method); if there was any suspicious lesion, biopsy was taken and in the absence of acetowhite epithelium, a random biopsy was taken from four cervical regions. If the colposcopy result was normal and satisfying, it was considered negative. For the abnormal cases or unsatisfactory colposcopy, biopsy or endocervical curettage (ECC) was performed and a sample was sent to the pathology department. If the pathological report indicated a CIN lesion or higher, it was considered a positive result. All cytology slides and biopsies or ECC samples were reviewed by a pathologist.
After collecting the data, frequency tables and statistical indices were mapped according to the background variables, together with the diagnostic value indices – including the specificity, sensitivity, positive and negative predictive values for the PAP smear results, colposcopy, biopsy and histopathological examination. The level of significance was considered lower than 0.05.
The main objective of this study was to determine the Pap smear test measured according to the correspondence between the biopsy and the histopathological results of the conizations or hysterectomies.
Results
In this study, 130 patients referred to the gynecologic clinic of the First Department of Obstetrics and Gynecology were evaluated, but 21 of the patients were excluded.
The patients included were between 22 and 75 years old, with an average age of 40 years old. Most of the patients (39%) fell inside the 30-39-year-old age group (Table 1).

The country side female patients were more affected compared to the patients from urban environments, smoking being often met among them.
Taking into consideration the results of the Pap smear tests, there were 21% of ASCUS cases, 17% of LSIL cases, 22% of HSIL cases, 35% of ASC-H cases, and 5% of AGC-NOS cases.
The colposcopies performed on the patients revealed higher-level lesions in 44% of the cases and lower-level lesions in 42% of the cases, the rest of the subjects showing negative results. Also, 76 out of 109 female subjects, included in the actual study, have undergone cervical biopsy as a result of a colposcopy. Among them, 50 patients had similar results on both biopsy and histological examinations (Table 2).
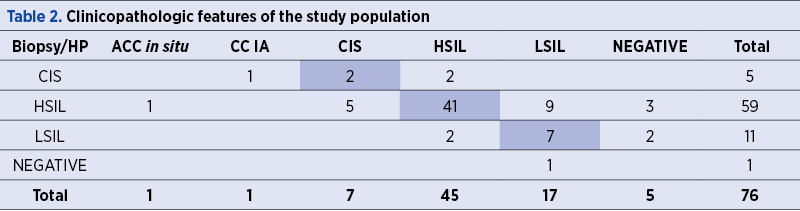
The HSIL and LSIL results obtained on the cytological examination and cervical biopsy were identical in 51% of the cases (20 patients); 41% of the cases were underdiagnosed and 8% overdiagnosed. The ASC-H, ASCUS and LSIL cervical cytology types were identified in 66 of the patients. The histopathological diagnosis after the biopsies confirmed CIN II in 23 subjects, CIN III in 19 patients, and CIS in 4 women.
Dividing the patients according to the treatment applied, 28.44% of them did not require surgical treatment, in comparison with 58.72% who have undergone a classic conization and 12.84% a total hysterectomy. Approximately a third of the patients needed a new surgical procedure (33% re-conization and 67% total hysterectomy).
Analyzing the correlations between histopathology and the cytology and taking into account only the LSIL and HSIL results, it has been proven that 16 patients (50%) had concordant results. However, the other half of them had different diagnosis; four patients were initially underdiagnosed and the histopathological results showed ACC in situ, together with squamous carcinoma stage IA and CIS (Table 3).
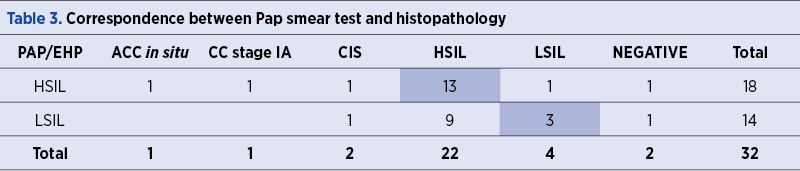
According to Table 4, the correlations between the conventional Pap smear test and biopsy showed a sensitivity of 86%, a specificity of 70%, a positive and negative predictive value of 92%, respectively 54%. On the other hand, the correlations between the Pap smear and the histopathological examination revealed some differences, a lower specificity (42%) and positive predictive value (76%); the sensibility and the negative predictive values were the same as the ones determined previously.
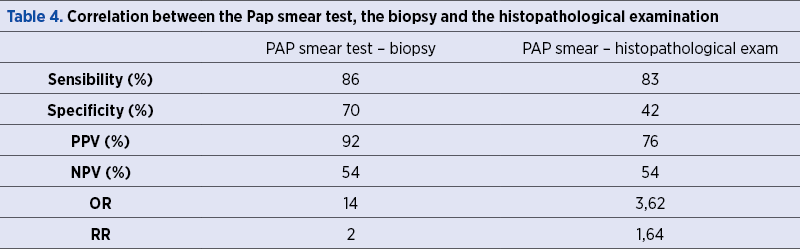
Taking into account the obtained data and using the Chi-square test, the existence of correlations between certain parameters was determined (Table 5).
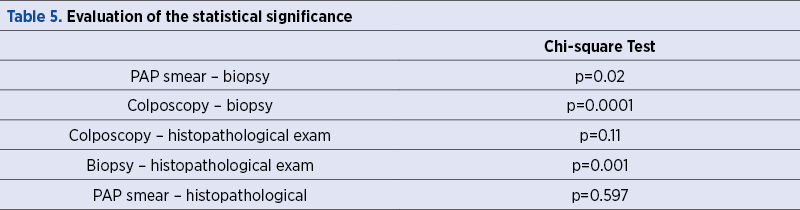
According to Table 5, we can observe a statistical correlation between the cytology-cervix biopsy, the colposcopy aspect of the lesions and the biopsy, the cervix biopsy and the histological result of conization and hysterectomy tissues. In contrast, it did not turn out to have any statistical significance, neither the correspondence between the cytology and the histopathological examination, nor the one between the colposcopic aspect of the lesions and the histopathological results.
Discussion
Cervical cancer is still representing a major public health priority. In at least a third of the countries, this type of cancer represents the most frequent neoplastic cause among women; in 90% of the countries, for the female patients under the age of 45, this type of cancer is one of the three neoplasia causes(8). Despite the remarkable progress in the prevention and treatment of cancerous lesions by associating the cytologic screening, HPV genotype and colposcopy(9), all together with prophylactic anti-HPV vaccination in developed countries like Australia, USA, The Netherlands or Denmark, the statistics in Romania are still disturbing, showing that the cervical neoplasia pathology represents the third cause of cancer in female patients(10).
The cervical cancer protection is the most important, with the screening being the main purpose, but as the prevalence of the disease decreases, other considerations may become equally important in the decision-making process.
An effective mass screening test – the Pap test – was introduced in the 1940s by George Papanicolaou and is based on the cytological morphology assessment of exfoliated cervical cells(11). An abnormal cytology report requires biopsy and histological confirmation.
The simplicity and the low cost are among the advantages of this technique. However, cytology-based cervical screening has some limitations also. The major problem is the low sensitivity of a single smear to detect high-grade precursor lesions (50-70%), which requires frequent testing(12). In addition, cytology has a low reproducibility, leading to variable accuracy(13). Moreover, by repeating cytology, the number of false-positive results increases substantially over time(14). Finally, the decrease in the incidence of cervical cancer induced by cytology-based screening is mainly restricted to squamous cell carcinoma, whereas no change is observed in the incidence of cervical adenocarcinoma, suggesting that cytology fails to detect adenocarcinomas and its precursors(15). Consequently, there is a need for a better primary screening test, and thus a new screening algorithm.
Nkwabong considers the use of biopsy in cases of relevant architectural macroscopic changes of the cervix, regardless of the result of the Pap test. Sensitivity, specificity, positive and negative predictive values of the smear tests were estimated to 55.5%, 75%, 88.2% and 33.3%(16).
Despite the fact that the performance of the exfoliative cytology has grown its capacity of indirect identification of cervical neoplasia, this has its own limits; improving the conventional Pap smear test, such as the liquid-based cytology and the electronic processing, revealed much more conclusive results(17).
Siebert and collaborators evaluated the long-term effectiveness of German cervical cancer screening with Pap testing. They reported the results for a homogeneous cohort of women with complete adherence to screening and follow-up(16). They consider that in the absence of screening, the model predicted a lifetime cervical cancer risk of 3%, with a peak incidence of 84/100,000 at the age of 51. Based on these decision analyses in the German healthcare context, annual Pap screening has the potential to prevent more than 98% of cervical cancer cases and more than 99% of deaths due to cervical cancer, resulting in an average gain in life expectancy of three months for each woman regularly participating in the screening program(18). These results were similar with the ones reported in health technology assessments conducted in the USA and UK(19).
A study developed by Petry and collaborators found out that the cytological examination has significant disadvantages. The rate of false-positive results is relatively high, 2-3% of all healthy participants in annual screening receiving an abnormal Pap smear result(20).
Human papillomavirus is considered the main etiological factor for the development of cervical intraepithelial neoplasia (CIN) and cervical cancer, since the viral DNA is detected in about 97% of cases(21).
A long-term cohort of 3406 HPV-negative women had 14.4% rate of false-positive cytology that led to unnecessary interventions and therapies. The surgical treatment of precursor lesions is associated with an increased risk of perinatal mortality and extreme premature delivery for subsequent pregnancies(22).
A meta-analysis of four of the six RCTs, published in Lancet, that included more than 176,000 participants with a subtle follow-up, including data of all national cancer registries, demonstrated a significantly improved prevention of invasive cervical cancer with HPV screening, reaching level I evidence(23).
In October 2020, the American Cancer Society has defined a new guideline for cervical cancer screening. This guideline has two goals; the primary goal is to detect treatable abnormalities and precancers (CIN2, CIN3, and adenocarcinoma in situ) and the secondary one, to determine the early-stage invasive cervical cancer, which also contributes to reducing mortality and decreasing treatment-related morbidity(24).
The new guidelines for cervical cancer screening recommend a primary HPV test alone every five years, starting at the age of 25. As an option, there are also admitted the Pap smear every three years or the cotesting every five years, if there is a limited or unavailable access to primary HPV testing. The population aged over 65 years old should continue screening until they meet one of the criteria: two consecutive, negative primary HPV tests, two negative cotests or three negative cytology tests in the past 10 years, the most recent done within the past 3-5 years. On the other hand, the individuals previously treated for histologic high-grade squamous intraepithelial lesions, CIN2, CIN3 or AIS should continue cervical cancer surveillance for at least 25 years(24).
A decision analysis for the US Preventive Services Task Force showed that starting the screening with primary HPV testing at the age of 25 retained over 99% of the life-years gained, with more cancer cases diagnosed and fewer colposcopies done, when compared to the strategy of cytology from the age of 21 followed by primary testing from 25 years(25).
According to the American Society of Colposcopy and Cervical Pathology, the management of cervical cancer screening abnormalities should be based on the risk, and not the results. The recommendations of colposcopy, treatment or surveillance will be based on the patients’ risk of CIN3+ determined by a combination of current results and past history (including unknown history). The same current test results may yield different management recommendations, depending on the history of recent past test results(24,26).
A unique screening experience of Kaiser Permanente of Northern California (KPNC)/National Cancer Institute Guidelines Cohort showed that the patients’ CIN3+ risk can be assessed based on current HPV and Pap smear test results and their recent history, colposcopic examination and the biopsy result, along with the treatments, all these by exploring the numerous potential risk factors(27).
The history of the HPV tests can alter the immediate risk and, therefore, the clinical management required. In consequence, a prior HPV-negative test estimates a lower risk than an unknown history, just like a negative HPV test followed by a positive one. On the other hand, a history of HPV-positive results leads to higher risk, even though the current test results are negative.
Among 1.5 million individuals who were included in the study, 92% had a primary HPV-negative test result at the first visit, corresponding to a 0.14% risk of developing CIN3+ within the following 5 years. If cotesting is used, it has been observed an HPV-negative result associated with an abnormal cytology (mainly ASC-US) in about 2% of the screened population. More important, only 0.01% of the individuals had HPV-negative HSIL+ and an immediate risk of CIN3+ of 25%. The screening interval can be extended to three years only after two cotests with negative result, because in this way the CIN3+ risk decreases to 0.29%. Most patients treated for CIN2 and CIN3 achieve a negative HPV test result on the first follow-up after the treatment, which leads to an immediate and 5-year CIN3+ risks of 0.34% and 2% resulting in one-year follow-up(27).
Conclusions
The cervical cancer screening through the Pap smear tests is a cheap and a very well developed system, but it is associated with a negative predictive value of around 50%. This limitation might be improved if taking into consideration more frequent checkups. HPV vaccination, introduced in 2006, seems to be a solution which reduces the risk of hrHPV infections and HSIL.
The future of cervical cancer screening towards which we are all heading refers to the HPV testing. The implementation of this method as a screening will be a revolution into the healthcare system, but will take some time until the population worldwide will benefit from it.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, et al. Cervical cancer: screening, diagnosis and staging. Journal of BUON. 2016:21(2):320-5.
- Moscicki AB, Schiffman M, Kjaer S, et al. Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl. 3):S3/42–S3/51.
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27.
- McCredie MRE, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–34.
- World Health Organization. Cervical cancer, human papillomavirus (HPV) and HPV vaccines: key points for policy-makers and health professionals; 2007.
- Bobdey S, Balasubramanium G, Kumar A, et al. Cancer screening: should cancer screening be essential component of primary health care in developing countries? Int J Prev Med. 2015; 6: 56.
- Karimi-Zarchi M, Zanbagh L, Shafii A, et al. Comparison of Pap smear and colposcopy in screening for cervical cancer in patients with secondary immunodeficiency. Electron Physician. 2015;7:1542–1548.
- Bosch FX. The path to eliminate cervical cancer in the world and the challenges of professional education. Vaccine. 2013;31 Suppl 7:ix-x.
- Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24 (suppl 3): S63-70.
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Romania – Human Papillomavirus and Related Cancer. Fact Sheet 2014. Available at: http://www.hpvcentre.net/statistics/reports/ROU_FS.pdf. Accessed: 16 Nov, 2015.
- Cannistra SA, Niloff JM. Cancer of the uterine cervix. N Engl J Med. 1996;334:1030–1038.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119: 1095–1101.
- Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132: 810–819.
- Katki HA, Wentzensen N. How might HPV testing be integrated into cervical screening? Lancet Oncol. 2012;13: 8–10.
- Bulk S, Visser O, Rozendaal L, et al. Cervical cancer in the Netherlands 1989-1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women. Int J Cancer. 2005;113:1005–1009.
- Nkwabong E, Badjan ILB, Sando Z. Pap smear accuracy for the diagnosis of cervical precancerous lesions. Tropical Doctor. 2019;49(1):34–39.
- Lonky NM. Cervical screening with in vivo and in vitro modalities: speculoscopy combined with cytology. In: Apgar BS, Brotzman GL, Spitzer M. Colposcopy: Principles and Practice, 2nd edition, Saunders Elsevier, Philadelphia, 2008, p. 91-99.
- Siebert U, Sroczynski G, Hillemanns P, Engel J, Stabenow R, Stegmaier C, et al. The German Cervical Cancer Screening Model: development and validation of a decision-analytic model for cervical cancer screening in Germany. European Journal of Public Health. 2006 Apr;16(2):185–192.
- Payne N, Chilcott J, McGoogan E. Liquid-based cytology in cervical screening: a rapid and systematic review. HTA Report: The National Coordinating Centre for Health Technology Assessment (NCCHTA); 2000. http://www.ncchta.org/execsumm/summ418.htm. Accessed August 19, 2005.
- Petry KU, Menton S, Menton M, Loenen-Frosch F, Gomes HC, Holz B, el al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. British Journal of Cancer. 2003;88(10):1570–1577.
- Lam JU, Rebolj M, Dugué PA, et al. Condom use in prevention of Human Papillomavirus infections and cervical neoplasia: Systematic review of longitudinal studies. J Med Screen. 2014;21(1):38-50.
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.
- Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. The Lancet. 2014;Vol.383;524-532.
- Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et el. Cervical Cancer Screening for Individuals at Average Risk: 2020 Guideline Update from the American Cancer Society. Cervical Screening for Average Risk.
- Kim JJ, Burger EA, Regan C, Sy S. Screening for Cervical Cancer in Primary Care. JAMA. 2018;320(7):706.
- Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102–31.
- Egemen D, Cheung LC, Chen X, Demarco M, Perkins RB, Kinney W, et al. Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020;24:132–143.
Articole din ediţiile anterioare
Formă severă de displazie bronhopulmonară – prezentare de caz
Displazia bronhopulmonară (DBP) este o afecţiune care apare în continuarea detresei respiratorii a prematurului. Boala afectează în special prematu...