Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm characterized by the presence of the Philadelphia chromosome. CML is classified into chronic, accelerated and blast phases. The blast crisis is characterized by the rapid expansion of a population of myeloid or lymphoid differentiation-arrested blast cells. Extramedullary blast crisis is defined as blast infiltration in other sites regardless of bone marrow blast proliferation. We report the case of a 59-year-old woman diagnosed with blast phase CML. Initially, the patient had an optimal response to tyrosine kinase inhibitors, but later she developed central nervous system blast crisis.
Criza blastică extramedulară în sistemul nervos central – entitate rară în leucemia cronică mieloidă
Extramedullar central nervous system blast crisis – rare occurrence in chronic myeloid leukemia
First published: 25 octombrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.56.3.2021.5649
Abstract
Rezumat
Leucemia mieloidă cronică (LMC) este o neoplazie mieloproliferativă cronică, fiind caracterizată de prezenţa cromozomului Philadelphia. LMC este clasificată în fazele cronică, accelerată şi blastică. Faza blastică se caracterizează prin proliferarea rapidă a unei populaţii de celule imature, oprite în diferenţiere, de linie mieloidă sau limfoidă. Criza blastică extramedulară este definită de infiltrarea în alte locusuri, indiferent de afectarea medulară. Raportăm cazul unei paciente în vârstă de 59 de ani, diagnosticată cu LMC în fază blastică. Iniţial, aceasta a avut un răspuns optim la tratamentul cu inhibitori de tirozin kinază, dar ulterior a dezvoltat o criză blastică cu determinare cerebrală.
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm characterized by a triphasic evolution, namely chronic, accelerated and blastic phases. Sometimes, the disease is diagnosed directly in the blast phase. Blast population higher than 20% in blood or/and bone marrow or an extramedullary blast proliferation are essential for the diagnose. In most cases (70%), the affected lineage is myeloid, while lymphoid lineage is encountered in 20-30% of cases(1). The mechanisms responsible for the transition of chronic-phase CML into blast crisis are known to be associated with BCR-ABL1 overexpression and secondary genes changes(2,3). Early and accurate identification of the blast phase may be difficult and can be delayed by the unusual pattern of the immature cells. Although the pathogenic effects of most CML blast crisis changes are still poorly understood, there is ample evidence that the phenotype of CML blast phase cells depends on the cooperation of BCR/ABL1 with dysregulated genes during disease progression(1-3).
Case presentation
We report the case of a 51-year-old female patient diagnosed with blast phase chronic myelogenous leukemia BRC-ABL1 p210 positive. The patient received chemotherapy (“3+7” regimen – cytarabine, idarubicin), started before obtaining the results of molecular and cytogenetic exams, then imatinib. The patient was eligible for allogeneic stem cell transplantation and the search for a matched donor was initiated.
After a month of TKI treatment, the patient developed nonhematological toxicity (arthralgia grade 3). Imatinib was stopped and the symptoms improved. The TKI was changed to dasatinib. No other significant adverse reactions were encountered. Therefore, the patient continued the TKI treatment, resulting in optimal response (BCR-ABL1 transcript 0.17% at six months after starting dasatinib), according to the ELN criteria (Figure 1).
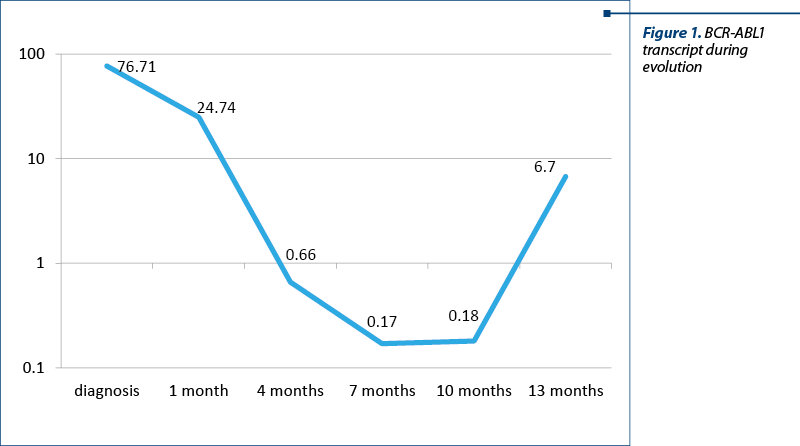
The patient developed severe SARS-CoV-2 pneumonia. After recovery, the transplant procedures were initiated.
One year after starting the treatment with dasatinib, the patient was admitted into the day clinic for a regular check-up. The clinical examination revealed no signs of disease progression or other complications. Nevertheless, the laboratory studies showed leukopenia (2.2 x 109/l). The peripheral blood smear showed no significant changes. At this time, the allogeneic stem cell transplantation was already scheduled. More important, the BCR-ABL1 transcript increased to 6.7% (Figure 1).
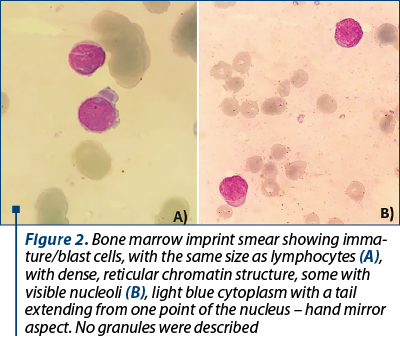
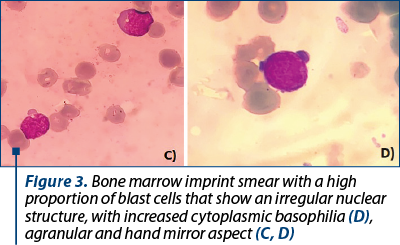
Shortly after molecular evaluation, the patient was admitted for severe anemia (hemoglobin 5.5 g/dl) and leucopenia with neutropenia (leukocytes 1.3 x 109/l, neutrophil count 0.2 x 109/l). No immature cells were seen on the peripheral blood smear. A bone marrow biopsy with imprint was performed. The bone marrow imprint smear displayed more than 20% blasts, with atypical morphology, with difficulties in establishing the lineage appurtenance by morphological exam. The blasts were small, with scanty cytoplasm, more like lymphoblasts. Some had very sparse azurophilic granules and vacuoles, but most of them had no granules (Figures 2 and 3).
Bone marrow aspirate was obtained for flow cytometry. Immunophenotyping of the blasts revealed a population of 44% myeloblasts cMPO-/+, HLA-DR+, CD117+, CD33+, CD3-, CD7-, CD13-/+, CD16-, CD11-, CD36-, CD105-, CD71+/-. Blood samples were collected for BCR-ABL mutation status screening.
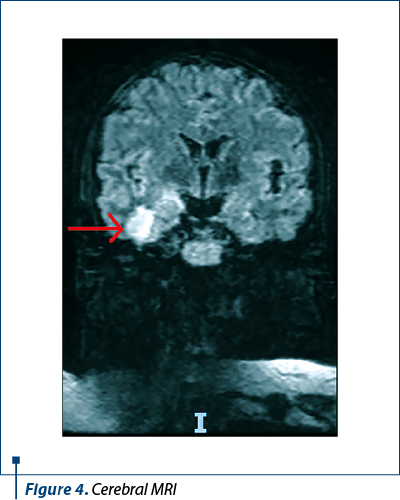
During hospitalization, the patient developed seizures. A CT scan was performed. However, it did not show any abnormalities. Therefore, the investigations continued with an MRI. Two nodular lesions were described, one in the hippocampus-amygdaloid complex and one in the inferior temporal gyrus, as shown in Figure 4.
Discussion
In transplant-eligible patients with blast crisis CML, the goal of treatment is to achieve the chronic phase and proceed to allogeneic stem cell transplantation as quickly as possible(4). In our case, the patient had an optimal response to TKI, but then she developed SARS-CoV-2 pneumonia, which prolonged the time to transplantation.
The COVID-19 pandemic has a negative impact on oncological patients, starting from the delay in diagnosis, difficulties in follow-up and delays in treatment, including stem cell transplantation. According to the American Society of Hematology, the disease and the treatment of CML do not need to change in the context of the COVID-19 pandemic(5). All CML patients should receive the SARS-CoV-2 vaccine unless other contraindications(5). The mortality rate in CML patients with COVID-19 infection was 13.7%. The adverse identified factors were older age and imatinib treatment(6).
The mechanisms of blast crisis in treated CML patients are not fully understood. It is generally believed that the persistence of BCR-ABL1 activity may cause genetic instability, leading to the acquisition of novel genetic abnormalities(7). Another hypothesis is based on the quiescent leukemia stem cells (LSCs). LSCs are resistant to TKI therapy and also difficult to assess by BCR-ABL1 qRT-PCR. Therefore, in CML patients treated with TKI who obtained a molecular response, the residual LSCs may be responsible for blast crisis(8). In our case, the patient had an optimal molecular response. Therefore, we suspect the involvement of a BCR-ABL1 non-related mechanism in blast crisis pathogenesis.
Extramedullary central nervous system blast crisis is a rare occurrence in CML patients. Many hypotheses for CNS leukemic infiltration were described, such as the expansion of LSCs into the perivascular spaces that reach into the brain, through the blood-brain barrier, blood-leptomeningeal barrier, blood-cerebrospinal fluid barrier, or through the dural lymphatic system(9).
Of all TKI inhibitors, dasatinib crosses the blood-brain barrier, being efficient in the management of CNS leukemic infiltration(10). In our case, at the time of CNS infiltration, the patient was already treated with dasatinib and she obtained an optimal molecular response.
Conclusions
The presence of latent LSCs in the bone marrow may be responsible for disease progression and TKI resistance independent of BCR-ABL1. Consequently, CNS disease can occur in patients treated with second-generation TKI dasatinib, even though an optimal molecular response was achieved.x
As a hallmark of peculiar transformation, the blast phase remains an unsolved and puzzling event.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103(11):4010–4022. doi:10.1182/blood-2003-12-4111.
-
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European Leukemia Net recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. doi:10.1182/blood-2013-05-501569.
-
Shi Y, Rand AJ, Crow JH, Moore JO, Lagoo AS. Blast Phase in Chronic Myelogenous Leukemia Is Skewed Toward Unusual Blast Types in Patients Treated withTyrosine Kinase Inhibitors: A Comparative Study of 67 Cases. American Journal of Clinical Pathology. 2015;143(1):105–119. doi:10.1309/AJCPWEX5YY4PHSCN.
-
Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737-747. doi: 10.1182/blood-2012-03-380147, doi:10.1182/blood-2012-03-380147.
-
Mauro M, Druker B, Radich J, Cortes J, Brümmendorf TH, Saglio G, et al. COVID-19 and CML: Frequently Asked Questions. Version 3.0. Last updated September 28, 2021. Available at: https://www.hematology.org/covid-19/covid-19-and-cml
-
Rea D, Mauro MJ, Cortes JE, Jiang Q, Pagnano KB, Ongondi M, et al. International CML Foundation; COVID-19 in Patients (pts) with Chronic Myeloid Leukemia (CML): Results from the International CML Foundation (iCMLf) CML and COVID-19 (CANDID) Study. Blood. 2020;136(1):46–47. doi:10.1182/blood-2020-140161.
-
Thielen N, Ossenkoppele GJ, Schuurhuis GJ, Janssen JJ. New insights into the pathogenesis of chronic myeloid leukaemia: towards a path to cure. Neth J Med. 2011;69(10):430-440.
-
Bocchia M, Sicuranza A, Abruzzese E, Iurlo A, Sirianni S, Gozzini A, et al. Residual Peripheral Blood CD26+ Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission. Front Oncol. 2018;8:194. doi: 10.3389/fonc.2018.00194.
-
Lenk L, Alsadeq A, Schewe DM. Involvement of the central nervous system in acute lymphoblastic leukemia: opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020;39(1):173-187. doi: 10.1007/s10555-020-09848-z.
-
Porkka K, Koskenvesa P, Lundán T, Rimpiläinen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. doi: 10.1182/blood-2008-02-140665.
Articole din ediţiile anterioare
Identificarea de noi markeri moleculari pentru predicţia răspunsului sau a recidivei în leucemia cronică mieloidă, la pacienţi trataţi cu inhibitori de tirozin kinază
Lucrarea reprezintă teza de doctorat a Dr. Ana Manuela Crişan. Cuprinde o parte generală, în care sunt aduse actualizări ale cunoştinţelor în domen...
Un caz interesant de leucemie mieloidă cronică manifestată sub formă de criză blastică limfoidă B
Prezentăm cazul unui pacient de 46 de ani, care s-a prezentat la camera de gardă în decembrie 2019 pentru durere în cadranul stâng abdominal.