Many studies characterize tumors biologically in order to emphasize their proliferative, invasive and metastatic abilities. The biological potential of malignant tumors is an important factor in their growth. The malignant phenotype is asociated with the combined action of some exo- or endopeptidases, such as cathepsin D. They constitute an enzymatic cascade which facilitates extracellular matrix degradation, an important stage in the invasion by tumor cells. In our study we applied a biochemical method to determine cathepsin D in the tumoral cytosol prepared from the tumor sampled between the 13th and 25th day. We noticed that the enzyme activity in the tumor tissue enhanced significantly (p<0.05) beginning with the 15th day and correlated the enzyme activity with the tumor growth and metastasis development parameters. The enhanced enzyme activity in the tumor tissue is consistent with the cathepsin D role in metastasis appearance and development.
Expresia catepsinei D în carcinosarcomul Walker-256 şi rolul ei în procesul de metastazare
Cathepsin D expression in Walker carcinosarcoma and its role in metastatic process
First published: 27 octombrie 2017
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.40.3.2017.1155
Abstract
Rezumat
Numeroase studii sunt destinate caracterizării biologice a tumorilor, pentru evidenţierea capacităţii lor de proliferare, invazie şi metastazare. Potenţialul biologic propriu tumorilor maligne este un factor important în evoluţia acestora. Fenotipul malign se asociază cu acţiunea concertată a unor proteaze exo-/endopeptidaze, printre care se numără şi catepsina D. Acestea formează o cascadă enzimatică prin care se facilitează degradarea matricei extracelulare, etapă importantă în invazia tumorală. În acest studiu am determinat printr-o metodă biochimică catepsina D în citosolul tumoral, obţinut din tumora primară recoltată între a 13-a şi a 25-a zi. În urma determinărilor biochimice s-a constatat o creştere semnificativă (p<0,05) a activităţii enzimei în ţesutul tumoral începând cu ziua a 15-a şi s-a corelat activitatea enzimei cu parametrii de evoluţie şi metastazare. Prezenţa în ţesutul tumoral a unei activităţi enzimatice crescute confirmă rolul catepsinei D în procesul de metastazare.
Introduction
Metastasis is one of the most important events in cancer pathogenesis, responsible for the most part of the human mortality through cancer.The development of sofisticated biochemical, immunological and genetic techniques that can be applied to spontaneous primary tumors and their metastases was an important stage in research and led to the detailed analysis of the tumoral progression, matastasis and therapy of the cancerous disease and its metastases. At present, it is well known that metastasis is dependent on both host’s characteristics and tumor’s characteristics.
For studying the experimental tumor metastases, it is certainly useful to have animal tumor models that mimic well the metastatic disease in humans. Some of these medels can have a limited usefulness and answer to some specific questions regarding the metastatic process, while others can be useful in studying a large area of problems related to cancer biology and metastases therapy.
Walker carcinosarcoma 256 is a tumor that appears spontaneously in the mammary glands of the rat, discovered by Walker. Initially, the tumor was diagnosticated as adenocarcinoma, in which structure there is also a sarcomatoid cell type population. Walker carcinosarcoma 256 is supported by subcutaneous grafts in Wistar rats, it has a metastatic potential, and the invasion of locoregional lymph nodes appears in the terminal phase of the tumoral growth, when tumor necrosis phenomena are accentuated. It is a standard tumor used in lymph node metastasis models, in the preclinical screening of the antitumor substances, and also in different experimental models of chemotherapy and radiation therapy.
The goal of our study was to elucidate the activity of cathepsin D, correlated with evolution and metastasis parameters.
Materials and method
Biological samplesThe study was conducted on 60 normal, healthy Wistar albino rats, with a 200 g medium weight, who were inoculated with 1,000.000 tumoral cells/ml, in the right side.
Cell viability was assessed using 0.1% Trypan Blue in TFS. Rats were sacrificed at 13, 15, 18, 21 and 25 days from grafts, when the primary tumor was evaluated and biologic tissue was sampled for obtaining the tumor cytosol. The local tumor was measured using two diameters, and the tumor volume was calculated using the following formula:
V = a x b² x 0.52 (a = the large diameter of the tumor; b = the small diameter of the tumor; 0.52 = thickness of the tegument).
Obtaining the cytosol
The homogenization of the tissue for isolating the cytosolic fraction was made using 10 mM Tris-HCl buffer, pH=7.4. The homogenate was centrifuged at 5000xg, for 10 minutes, and the supernatant was ultracentrifuged at 100000xg for one hour.
Determinations
The cytosolic protein concentration was determined by two methods, Lowry and Bradford, with bovine serum albumin (BSA) as standard. The method used for the biochemical determination of the cathepsin D was similar to the method described by Capony. The reaction took place in Acetate Buffer 1M pH 3.5, with 4% hemoglobin as substrate, and it was stopped with 2 ml of 3% trichloroacetic acid.
A standard curve was made, for whose plotting we used pure cathepsin D, obtained from Sigma company. For control, 20 µl of pepsatin A were added to the reaction, resulting a final concentration of 10-6 M. A second control sample was realized, adding the tumor extract followed by the trichloroacetic acid, which stopped the reaction.
Results and discussion
Walker carcinosarcoma 256 had a good growth, the tumor became palpable at 8-14 days after the inoculation, and the micrometastases were present in the liver. At 15-21 days after the inoculation we could notice hepatic macrometastases. In the final phases, they covered the entire liver surface and after 7 days the rat’s mortality from our study was almost 100%.
The evolution and metastatic process of the Walker carcinosarcoma 256 were assessed by measuring the tumor volume and by the quantitative evaluation of the hepatic tumors.
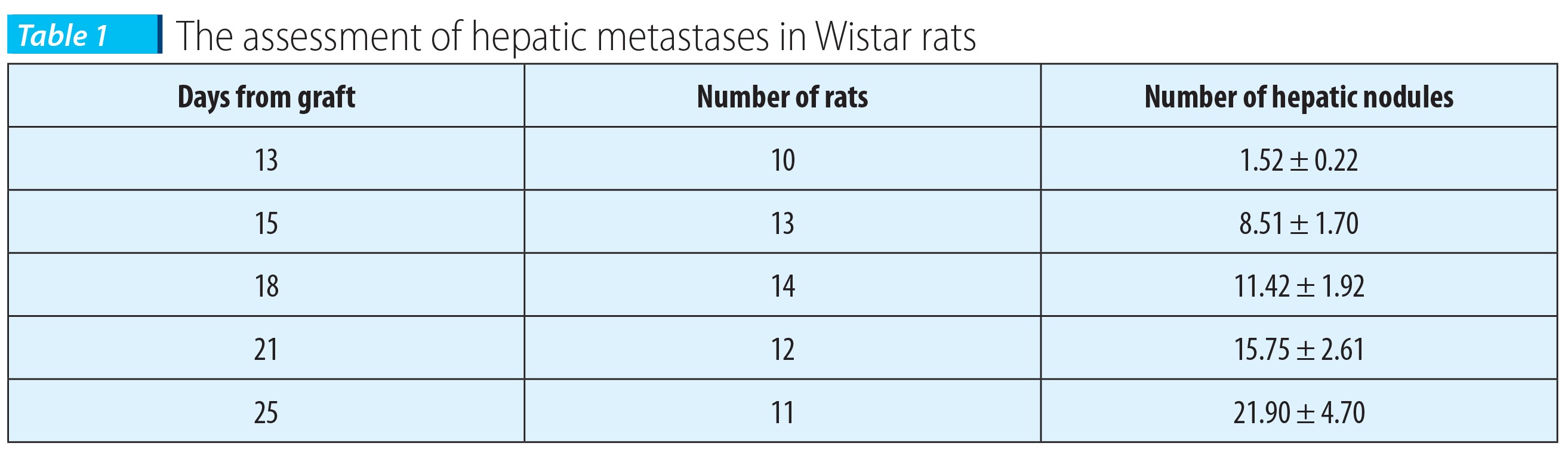
Table 1 presents the evaluation of hepatic metastases, in the evolution course of Walker carcinosarcoma 256, in Wistar rats subcutaneously grafted with 1x106 living cells.
The results presented in Table 1 are means obtained from two lots grafted at different moments, in which the rats presented quite similar evolutions.
The tumor volume was significantly increased (p<0.05) in the 18th day versus the 13th day, and the metastasis parameters were significantly different (p<0.05) from those from the 13th day and even from those from the 15th day.
The 15th day can be considered the moment of the obvious appearance of liver metastases in our experimental conditions.
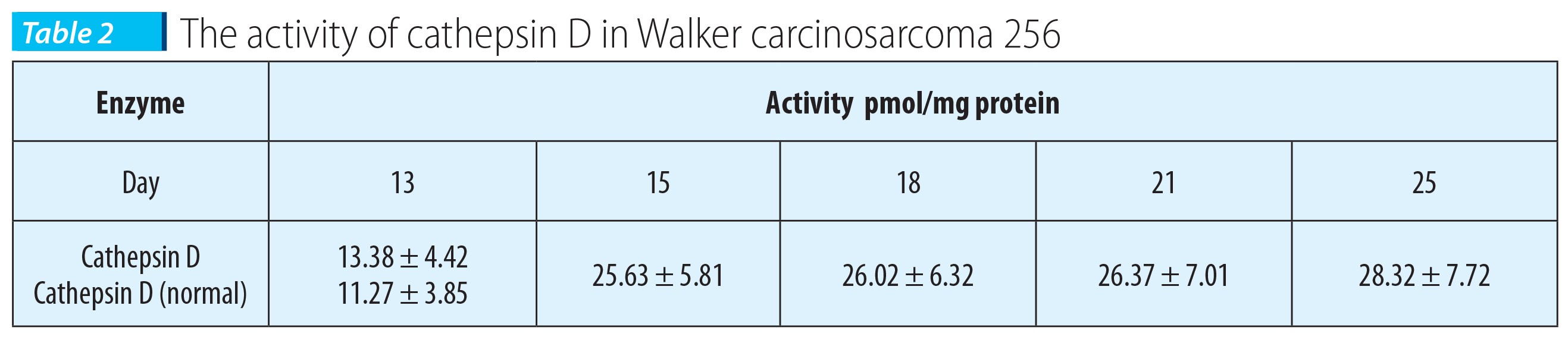
From the primary tumor sampled in days 13, 15, 18, 21 and 25 it was prepared the tumor cytosol in which there were dosed the cytosolic protein and the cathepsin D. The activity of cathepsin D was expressed in pmoli/mg cytosolic protein.
The results presented in Table 2 indicate the modification of this degradative enzyme in the primary tumor, subcutaneously grafted, in the process of enzyme formation.
The activity of cathepsin in the primary tumor in the 13th day doesn’t differ significantly from normal. A significant increase of the enzyme activity was noticed in the 15th day, compared to the activity measured at 13 days after inoculation. The activity measured in days 18, 21 and 25 doesn’t differ significantly from the one measured in the 15th day. The differences in the cathepsin D activity, in the primary tumor, in days 15, 18, 21 and 25 are elevated, with statistical significance.
Cathepsin D activity is 2.12 times higher in the 25th day compared to the 13th day. The presence in the tumoral tissue of an enhanced enzymatic activity of cathepsin D, in a metastasizing tumor such as Walker carcinosarcoma 256, is in agreement with the presumed role of cathepsin D in the metastasis process.
Conclusions
It was noticed a positive correlation between the growth time of lysosomal enzyme, cathepsin D, in Walker carcinosarcoma 256, and the appearance of micro- and macrometastases in liver.The mechanisms responsible for the intratumoral lysosomal enzyme growth are unidentified, but they might be related to macrophage infiltration and other tumor-host interactions, which can facilitate the tumor cell disemination. Our results suggest that the inhibition of this enzyme may open the way for new therapeutic methods for controlling the malignant disease.
Bibliografie
2. Miscalencu D, Nicolae Ilinca, Mailat Florica, Szegli Gheza, Szegli Gheza, Schipor Sorina. Factori moleculari ai metastazării tumorale, Ed. Universităţii Bucureşti, 2004.
3. G. Weber. Molecular Mechanism of Cancer, Springer, 2007.
4. Olinici CD. Biologia celulară a cancerului, Ed. Medicală, Bucureşti, 2010.
5. Mona Mostafa Mohamed, Bonnie F. Sloane. Cysteine cathepsins: multifunctional enzymes in cancer, Nature publishing Group, Vol. 6, 2006.
6. Sajid M & McKerrow JH. Cysteine proteases of parasitic organisms. Mol Biochem. Parasitol, 2002, 120, 1-21.
7. McKerrow JH. Development of cysteine protease inhibitors as chemotherapy for parasitic diseases: insights on safety, target validation, and mechanism of action. Int. J. Parasitol, 1999, 29, 833–7.
8. Saftig P et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci, 1998, USA 95, 13453–8.
9. Shi GP et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10, 1999, 197–206.
10. Roth W et al. Cathepsin L deficiency as molecular defect of furle hyperproliferation of keratinocyte sand pertubation of hair follicle cycling. FASEB J., 2000, 14, 2075–86.
11. Stypmann J et al. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc. Natl Acad. Sci, 2002, USA 99, 6234–9.
12 Reinheckel T, Deussing J, Roth W & Peters C. Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 382, 2001, 735–41.
13. Driessen C, Lennon-Dumenil AM & Ploegh HL. Individual cathepsins degrade immune complexes internalized by antigen-presenting cells via Fcγ receptors. Eur. J. Immunol., 2001, 31, 1592–601.
14. Felbor U et al. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci., USA, 2002, 99, 7883–8.
15. Pham CT, Ivanovich JL, Raptis SZ, Zehnbauer B & Ley TJ. Papillon. Lefevre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J. Immunol., 2004, 173, 7277–81.
16. Pham CT & Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc. Natl Acad. Sci. USA, 1999, 96, 8627–32.
17. Caughey GH. New developments in the genetics and activation of mast cell proteases. Mol. Immunol., 2002, 38, 1353–7.
18. Xia L et al. Localization of rat cathepsin K in osteoclasts and resorption pits: inhibition of bone resorption and cathepsin K-activity by peptidyl vinyl sulfones. Biol. Chem., 1999, 380, 679–87.
19. Friedrichs B et al. Thyroid functions of mouse cathepsins B, K, and L, J. Clin. Invest., 2003, 111, 1733–45.
20. Hughes SJ et al. A novel amplicon at 8p22–23 results in overexpression of cathepsin B in esophageal adenocarcinoma. Proc. Natl Acad. Sci., USA, 1998, 95, 12410–5.
21. Lin L et al. A minimal critical region of the 8p22–23 amplicon in esophageal adenocarcinomas defined using sequence tagged site-amplification mapping and quantitative polymerase chain reaction includes the GATA-4 gene. Cancer Res., 2000, 60, 1341–47.
22. Berquin IM, Cao L, Fong D & Sloane BF. Identification of two new exons and multiple transcription start points in the 5′-untranslated region of the human cathepsin-B-encoding gene. Gene, 1995, 159, 143–9.
23. Seth P, Mahajan VS & Chauhan SS. Transcription of human cathepsin L mRNA species hCATL B from a novel alternative promoter in the first intron of its gene. Gene 321, 2003, 83–91.
24. Arora S & Chauhan SS. Identification and characterization of a novel human cathepsin L splice variant. Gene, 2002, 293, 123–31.
25. Yan S & Sloane BF. Molecular regulation of human cathepsin B: implication in pathologies. Biol. Chem., 2003, 384, 845–54.
26. Georgescu D, Comisel V, Enăchescu F. Oncobiologie, Ed. Ars Docendi, Bucureşti, 2000.