Objectives. Bone tumors are heterogeneous neoplasms, comprising a large spectrum of entities, both benign and malignant. One of the greatest challenges regarding bone and soft tissue pathology is highlighting osteoblastic differentiation in malignant lesions. From the numerous osteoblast-specific markers previously described in the literature, only osteocalcin, osteonectin and RANK have been used in histology studies. More recent proposed markers for osteoblastic differentiation are CD56 and special AT-rich sequence binding protein 2 (SATB2). Giant cell tumour of the bone is a special entity, in the category of intermediate tumors, locally aggressive and rarely metastasising. It is also known to become malignant. Specific markers for giant tumor of the bone are p63 and RANK, the latter being an osteoblastic marker. We investigated the expression of the quoted recent emerged markers, p63, CD56 and SATB2, in 23 bone and soft tissue primary and secondary neoplasms by constructing a tissue microarray paraffin block, and we analysed the impact of using them as a diagnostic marker in current practice.
Expresia imunohistochimică a SATB2 în tumorile osoase, cu accent pe tumora de celule gigantice şi malignităţi
SATB2 immunohistochemical expression in bone tumors with emphasis on giant cell tumor and malignancy
First published: 29 decembrie 2018
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.45.4.2018.2166
Abstract
Rezumat
Obiective. Tumorile osoase sunt neoplasme eterogene, cuprinzând un spectru larg de entităţi, atât benigne, cât şi maligne. Una dintre cele mai mari provocări privind patologia osoasă şi a ţesuturilor moi evidenţiază diferenţierea osteoblastică în leziunile maligne. Dintre numeroşii markeri osteoblastici specifici descrişi anterior în literatură, numai osteocalcinul, osteonectina şi RANK au fost utilizaţi în studiile de histologie. Markerii mai recenţi propuşi pentru diferenţierea osteoblastică sunt CD56 şi proteina 2 de legare în secvenţa ATrich specială (SATB2). Tumorile cu celule gigantice ale oaselor sunt o entitate specială, în categoria tumorilor intermediare, la nivel local agresiv şi rareori cu metastaze. Este, de asemenea, cunoscut faptul că pot deveni maligne. Markerii specifici pentru tumora gigantică a osului este p63 şi RANK, acesta din urmă fiind un marker osteoblastic. Am investigat expresia markerilor recurenţi, p63, CD56 şi SATB2, în 23 de neoplasme primare şi secundare ale cancerelor osoase şi ale ţesuturilor moi prin construirea unui bloc de parafină microarray şi am analizat impactul utilizării acestora ca marker de diagnostic în practica curentă .
Introduction
In bone and soft tissue pathology practice, diagnosing malignant tumors like osteosarcoma is a difficult task, especially in biopsy samples(1). But an accurate decision is crucial, as chemotherapy and surgery approaches are fundamentally distinct for different tumor types(2-4).
Immunohistochemistry has been assessed as an additional tool to the morphological investigation, and according to diagnostic immunohistochemistry, a series of osteoblast-specific markers have been described in the literature, such as osteocalcin, osteonectin, bone morphogenetic protein (BMP), COL-I-C peptide, bone sialoprotein, bone glycoprotein, 75 decorin, osteopontin, proteoglycans I and II, type I collagen and RANK, but only osteocalcin and osteonectin have been used in diagnostic immune histologic studies, without a special resonance in clinical practice(5).
But in the last decade a new series of antibodies has been being proposed as osteoblastic differentiation markers, such as RANK(6,7), CD56(8) and SATB2(9).
CD56 is a homophilic binding glycoprotein with a role in cell-cell adhesion. As an IHC marker, its main uses are in hematopoietic disorders, as a marker of NK cells, for detecting residual myeloma or differentiate plasma cells in myeloma(10) from reactive plasmacytosis. Hughes, based on previous evidence of CD56 expression in osteoblast lineage, presented a study which concluded that CD56 may be a biologically important molecule in osteoblast function, and its expression is consistently strong in normal and neoplastic osteoblasts.
In 2012, Kallen detected p63 expression in osteoblastic tumors(11). Previously, p63 had been known as a member of p53 gene family(12), but without being a tumor suppressor gene. It appeared to regulate the development of epithelial organs and may be a molecular switch for the initiation of “epithelial stratification program”, regulating human keratinocyte proliferation. Isoform deltaNp63 inhibits the transcription activation of p53 gene(13).
Then, in 2013, Hornick suggested SATB2 as a potential marker for osteoblastic differentiation in bone and soft tissue tumors. It encodes at least six different proteins with different biologic functions(9).
SATB2 – known as special AT-rich sequence-binding protein 2 – is an AT-rich DNA-binding protein that binds to nuclear matrix attachment regions involved in transcriptional regulation and chromatin remodeling(14).
Initial studies showed that SATB2 protein is expressed in colon and rectum tissue and tumors(15), brain, branchial arches and osteoblast-lineage cells(16,17), kidney, bladder(18), some lymphoid cells and ovarian tumors(19). Despite been identified in multiple tissues, it has not been thoroughly investigated as a potential neoplastic marker in bone and soft tissue tumors.
Our purpose was to study the potential diagnostic applications of the three quoted antibodies as immunohistochemical markers in bone and soft tissue pathology, as well as evaluating positive expression scores and artefactual staining patterns.
Materials and method
We searched the Foişor Hospital database, for all the bone and soft tissue tumors from 2015 to 2018 and encountered 39 cases (Table 1 and Table 2). The cases were extracted according to the year of diagnosis, histological diagnosis, localization, and included data on gender, age of patients, site, morphological features, and the presence or absence of the primary tumor in patient’s history.
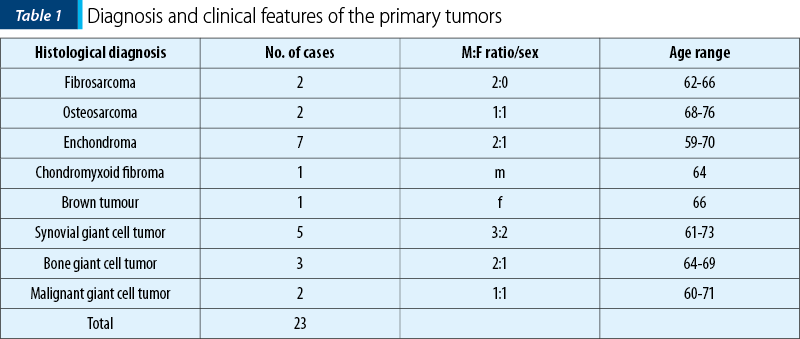
The tumors were subtyped according to diagnosis in 23 primary bone tumors (benign and malignant) as follows (Figure 1): two fibrosarcomas, two osteosarcomas, seven chondromas, ten giant cell tumors (GCT; two of them malignant), one chondromyxoid fibroma, one brown tumor; and 16 metastatic tumors: two lung carcinomas, five clear cell renal carcinoma, one prostate carcinoma, one colon adenocarcinoma, one hepatocarcinoma, two breast carcinomas, four unknown primary adenocarcinomas.

The hematoxylin-eosin slides for all the cases were reexamined, by three independent pathologists. We used Olympus CX41 microscopes equipped with digital Colorview III cameras for the optical examination, and for the morphological and comparative study, the Virtual Slide Microscope VS120 and the viewing software.
We constructed two tissue microarray paraffin blocks (TMA) using the technique(20,21), as follows (Figure 2). All donor blocks for the study cases have been selected and the region of interest defined on the paraffin wax block. After the initial review and selection, the donor blocks were arranged and kept in the order that is represented in the TMA. The array construction was made manual and was accompanied by a computer file, containing the tissue block identification numbers. The TMA was divided into four rows designated by letters and six columns ordered by numbers. Two-mm diameter donor tissue core needles were sampled. Tissue cores were punched from the predefined region, and then transferred to a recipient paraffin wax block, into a readymade hole. The obtained paraffin blocks were incubated and processed as usual (Figure 3).
For the immunostaining, we followed the indirect protocol for paraffin embedded tissue sections. The tissue section slides were deparaffinized, rehydrated and incubated with a 3% hydrogen peroxide solution, and heat induced antigen retrieval (HIER). For the staining we used Avidin-Biotin Complex (ABC) method and counterstaining with Mayer hematoxylin.
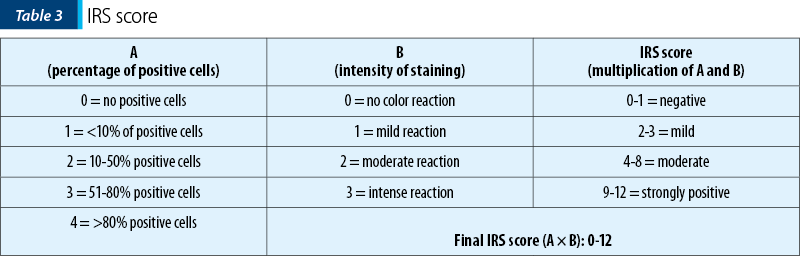
For the IHC expression we used the immunoreactive score (IRS)(22). The immunoreactive score (IRS) uses a range of 0-12 as a product of multiplication between positive cells proportion score (0-4) and staining intensity score (0-3) (Table 3). The immunoreactive score was broadly used for the expression of a wide spectrum of IHC markers(23-25).
Results
We evaluated the expression of p63, CD 56 and SATB2 on the 39 tissue samples.
The intensity of the reaction was variable, positive expression intensity ranging from weak signal (1) to strongly positive (3).
Using IHC, we found that SATB2 positive expression was highly restricted to nuclei. SATB2 was not detected in any adult normal non-osteoblastic tissue. Instead, we had experimented cases with nucleolar artefactual staining. In bone forming neoplastic tissue, SATB2 was detectable by IHC in 70% of 23 primary bone neoplasms examined.
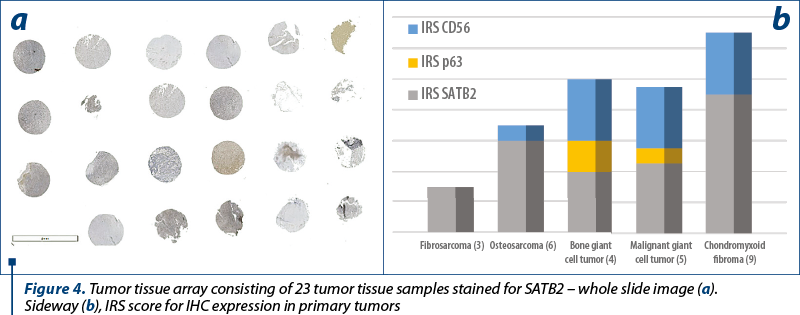
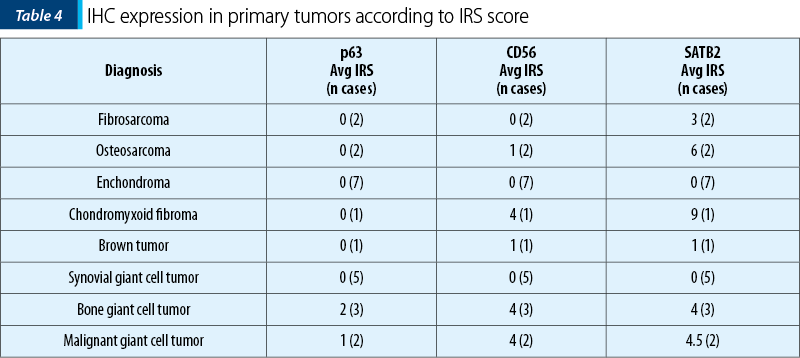
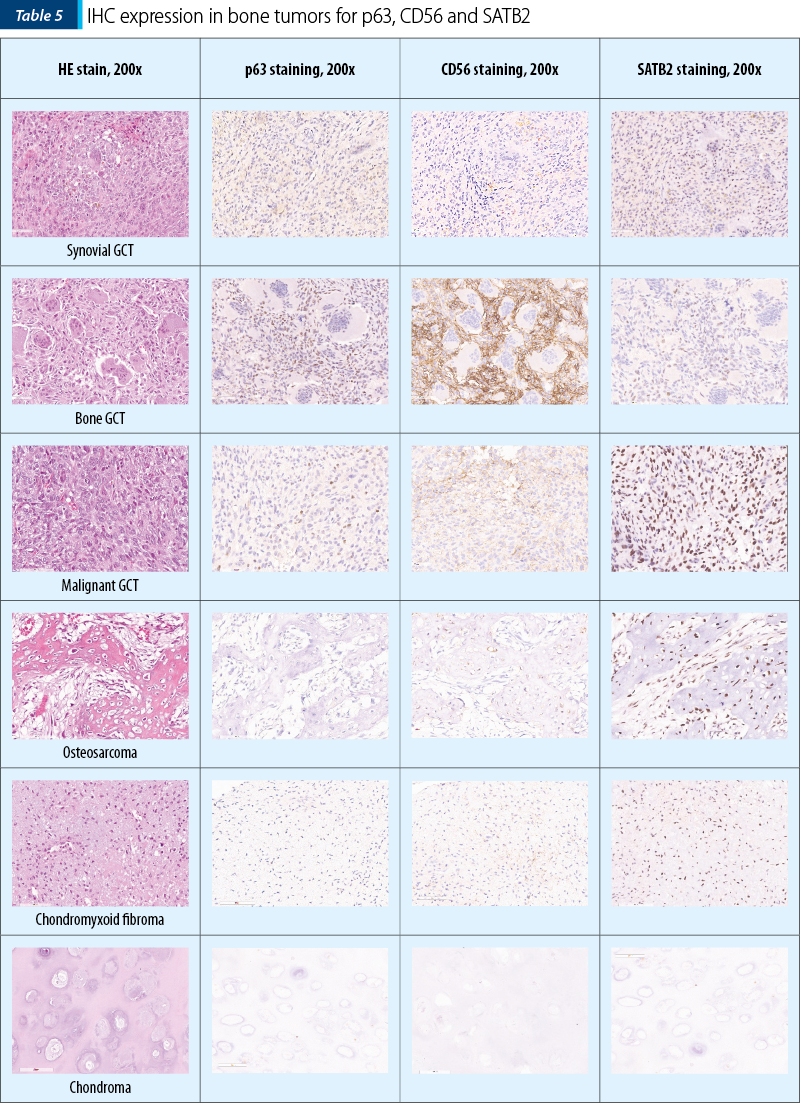
The two osteosarcomas were negative for p63 and one presented moderate expression for CD56, while for SATB2 we noted moderate expression in ~55% (30-80%) of the tumor cells, in both cases. Remarkably, the fibrosarcoma tumors showed moderate expression in 40% of the tumor cells for SATB2 and negativity for p63 and CD56. Synovial giant cell tumor didn’t show any reactivity to the antibodies, while the bone counterpart both malignant and benign ones showed the highest percentage of stained cells, but the intensity of the signal was weak in the benign tumors and strong in the malignant ones. Chondroid tumors didn’t show any positive staining for SATB2 or p63 and CD56 (Table 4 and Table 5).
Of the 16 metastatic tumors (two lung carcinomas, five clear cell renal carcinoma, one prostate carcinoma, one colon adenocarcinoma, one hepatocarcinoma, two breast carcinomas, four unknown primary adenocarcinomas in our study), only the colorectal carcinoma showed significant labeling of the nuclear region, thus confirming the literature data(26).
Discussion and conclusions
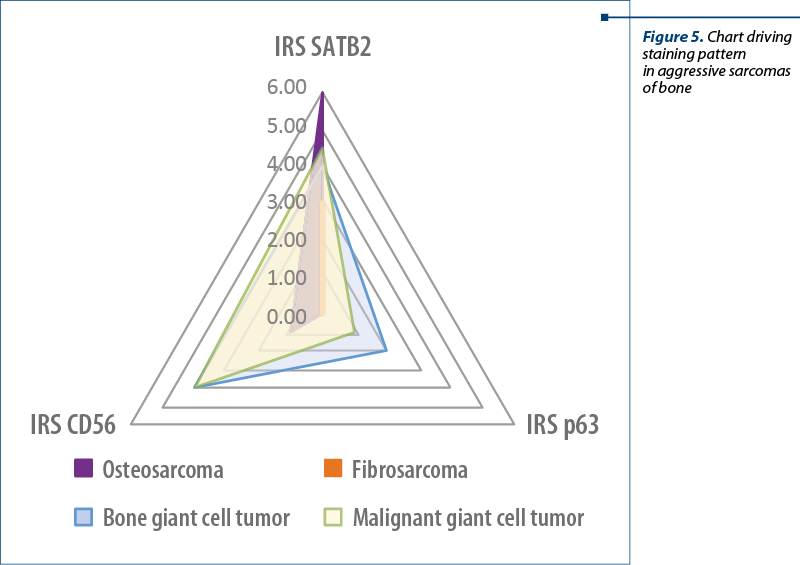
The aim of the study was to compare the potency of markers to fulfill an objective classification of osteoblastic differentiation in mesenchymal tumors. Of these, SATB2 showed the highest numbers of cells marked and the highest intensity of the signal, while the CD56 showed the lowest numbers of marked cells. P63 lack of reactivity with high grade osteosarcoma makes it useful only for bone giant cell tumors and, when needed, as an indirect control for SATB2 positive tumors.
While SATB2 can be considered neither a sensitive marker nor a specific one, it can become a valuable tool in diagnosing mesenchymal tumors in association with p63 and CD56. As from Table 4 and Figure 5, using p63 is highly valuable in IHC staining diagnosis of giant cell tumors, while SATB2 and CD56 are more useful in the diagnosis of aggressive osteosarcomas and malignancy in giant cell tumors.
One bizarre SATB2 staining pattern was observed in several cases showing nucleolar/dot like fashion staining. The aspect was mentioned before in literature, but it was not investigated to our knowledge. The presence of a homogeneous nuclear staining pattern associated with a nucleolar staining pattern in the same tumor suggests that this should not be considered a mere artifact and deserves further investigation.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Biazzo A, De Paolis M. Multidisciplinary approach to osteosarcoma. Acta Orthop Belg. 2016 Dec; 82(4):690-8.
- Moriceau G, Ory B, Gobin B, Verrecchia F, Gouin F, Blanchard F, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010; 16(27):2981-7.
- Verron E, Schmid-Antomarchi H, Pascal-Mousselard H, Schmid-Alliana A, Scimeca J-C, Bouler J-M. Therapeutic strategies for treating osteolytic bone metastases. Drug Discov Today. 2014 Sept; 19(9):1419-26.
- Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018; 18(1):39-50.
- Elsevier: Diagnostic Immunohistochemistry, 5th Edition: Dabbs [Internet]. [15 January 2019]. Valabil la: https://www.elsevier.ca/ca/product.jsp?isbn=9780323477321
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 15 May 2008; 473(2):139-46.
- Zhang S, Wang X, Li G, Chong Y, Zhang J, Guo X, et al. Osteoclast regulation of osteoblasts via RANK‑RANKL reverse signal transduction in vitro. Mol Med Rep. 2017 Oct; 16(4):3994-4000.
- Ely SA, Knowles DM. Expression of CD56/Neural Cell Adhesion Molecule Correlates with the Presence of Lytic Bone Lesions in Multiple Myeloma and Distinguishes Myeloma from Monoclonal Gammopathy of Undetermined Significance and Lymphomas with Plasmacytoid Differentiation. Am J Pathol. 2002 April; 160(4):1293-9.
- Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013; 63(1):36-49.
- Ceran F, Falay M, Dağdaş S, Özet G. The Assessment of CD56 and CD117 Expressions at the Time of the Diagnosis in Multiple Myeloma Patients. Turk J Haematol Off J Turk Soc Haematol. 2 August 2017; 34(3):226-32.
- Kallen ME, Sanders ME, Gonzalez AL, Black JO, Keedy VL, Hande KR, et al. Nuclear p63 expression in osteoblastic tumors. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2012 Oct; 33(5):1639-44.
- El Husseini N, Hales BF. The Roles of P53 and Its Family Proteins, P63 and P73, in the DNA Damage Stress Response in Organogenesis-Stage Mouse Embryos. Toxicol Sci Off J Soc Toxicol. 1 April 2018; 162(2):439-49.
- Yamano S, Gi M, Tago Y, Doi K, Okada S, Hirayama Y, et al. Role of deltaNp63(pos)CD44v(pos) cells in the development of N-nitroso-tris-chloroethylurea-induced peripheral-type mouse lung squamous cell carcinomas. Cancer Sci. 2016 Feb; 107(2):123-32.
- SATB2 SATB homeobox 2 [Homo sapiens (human)] - Gene - NCBI [Internet]. [15 January 2019]. Available at la: https://www.ncbi.nlm.nih.gov/gene/23314
- Berg KB, Schaeffer DF. SATB2 as an Immunohistochemical Marker for Colorectal Adenocarcinoma: A Concise Review of Benefits and Pitfalls. Arch Pathol Lab Med. 2017 Oct; 141(10):1428-33.
- Leone DP, Heavner WE, Ferenczi EA, Dobreva G, Huguenard JR, Grosschedl R, et al. Satb2 Regulates the Differentiation of Both Callosal and Subcerebral Projection Neurons in the Developing Cerebral Cortex. Cereb Cortex N Y N 1991. 2015 Oct; 25(10):3406-19.
- Zhao X, Qu Z, Tickner J, Xu J, Dai K, Zhang X. The role of SATB2 in skeletogenesis and human disease. Cytokine Growth Factor Rev. 2014 Feb; 25(1):35-44.
- Giannico GA, Gown AM, Epstein JI, Revetta F, Bishop JA. Role of SATB2 in distinguishing the site of origin in glandular lesions of the bladder/urinary tract. Hum Pathol. 2017; 67:152-9.
- Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011 July; 35(7):937-48.
- Jawhar NMT. Tissue Microarray: A rapidly evolving diagnostic and research tool. Ann Saudi Med. 2009 Apr; 29(2):123-7.
- Fernebro E, Dictor M, Bendahl P-O, Fernö M, Nilbert M. Evaluation of the tissue microarray technique for immunohistochemical analysis in rectal cancer. Arch Pathol Lab Med. 2002 June;126(6):702-5.
- Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathol. 1987 May; 8(3):138-40.
- Specht E, Kaemmerer D, Sänger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015 Sept; 67(3):368-77.
- Wróel T, Mazur G, Dziegiel P, Jelen M, Szuba A, Kuliczkowski K, et al. Density of intranodal lymphatics and VEGF-C expression in B-cell lymphoma and reactive lymph nodes. Folia Histochem Cytobiol Pol Acad Sci Pol Histochem Cytochem Soc. 1 February 2006; 44:43-7.
- Geis C, Fendrich V, Rexin P, Di Fazio P, K Bartsch D, Ocker M, et al. Ileal neuroendocrine tumors show elevated activation of mammalian target of rapamycin complex. J Surg Res. 2015 Apr; 194(2):388-93.
- Dragomir A, de Wit M, Johansson C, Uhlen M, Pontén F. The Role of SATB2 as a Diagnostic Marker for Tumors of Colorectal Origin: Results of a Pathology-Based Clinical Prospective Study. Am J Clin Pathol. 1 May 2014; 141:630-8.