Background. Bevacizumab therapy, indicated in the treatment of metastatic colorectal cancer, although associated with an increased overall survival, is frequently accompanied by certain complications that raise particular issues from a surgical point of view. Among these, gastrointestinal and complex colovaginal fistulas can raise severe problems in the surgical management of the case. Case presentation. A 43-year-old patient, diagnosed with lower rectal neoplasm, stage cT3N1M1PUL – stage IV (histopathological exam: ADK G2), followed, as a first stage of the multimodal treatment, 28 sessions of external radiotherapy associated with capecitabine. Under this treatment, numerical and dimensional progression of M1PUL was observed, therefore it was decided to continue chemotherapy in combination with bevacizumab. The subsequent evolution of M1PUL was favorable, the patient being referred to the surgery department where rectosigmoid resection was performed via the anterior route, followed by mechanical colorectal anastomosis and protective ileostomy. Despite the ileostomy, the patient developed an anastomotic fistula with a small flow which was treated conservatively until it was closed. After closing the ileostomy, due to the progression of M1PUL, it was decided to resume the therapy with the VEGF inhibitor, with the appearance of a complex colovaginal fistula, documented clinically and by imaging. Discussion. Its treatment involved the de facto definitive abolition of the mechanical colorectal anastomosis, in order to put the fistula to rest and allow for the reduction of local inflammation, with the subsequent resolution of the fistulous path. Conclusions. Although our case does not present rare complications of bevacizumab therapy, it highlights certain surgical problems that must be taken into consideration when initiating/resuming therapy. Although there are surgical solutions, in the case of our patient, the problem of permanent mutilation occurred in a young person. This raises the question of whether bevacizumab therapy should not be reconsidered in certain patients, considering that, in the absence of an overall survival benefit, the physical and mental discomfort that occurs as a result of the specific complications of this therapy may weigh more than the mental comfort given by the absence of neoplastic progression. Of course, this statement requires further studies that should evaluate the impact of these complications on patients.
Fistula complications of bevacizumab therapy in metastatic colorectal cancer – oncology surgeon’s point of view: a case presentation
Complicaţiile fistulare ale terapiei cu bevacizumab în cancerul colorectal metastatic – punctul de vedere al chirurgului oncolog: prezentare de caz
First published: 13 decembrie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.61.4.2022.7414
Abstract
Rezumat
Context. Terapia cu bevacizumab, indicată în tratamentul cancerului colorectal metastatic, deşi asociată cu o supravieţuire globală crescută, este frecvent însoţită de anumite complicaţii care ridică probleme deosebite din punct de vedere chirurgical. Printre acestea, complicaţiile fistulei gastrointestinale şi cele complexe colovaginale pot ridica probleme grave în managementul chirurgical al cazului. Prezentarea cazului. Un pacient de 43 de ani, diagnosticat cu neoplasm rectal inferior, stadializat cT3N1M1PUL – stadiul IV (examen histopatologic: adenocarcinom moderat diferenţiat), a urmat ca primă etapă a tratamentului multimodal radioterapie externă de 28 de şedinţe asociate cu capecitabină. În cadrul acestui tratament, s-a observat progresia numerică şi dimensională a M1PUL, motiv pentru care s-a decis continuarea chimioterapiei în asociere cu bevacizumab. Evoluţia ulterioară a M1PUL a fost favorabilă, pacientul fiind îndrumat către departamentul de chirurgie oncologică, unde s-a efectuat rezecţia rectosigmoidiană pe cale anterioară urmată de colorectoanastomoză mecanică şi ileostomie de protecţie. În ciuda ileostomiei, pacientul a dezvoltat o fistulă anastomotică cu debit scăzut, care a fost tratată conservator până la închidere. La şase luni de la închiderea ileostomiei, din cauza progresiei M1PUL, s-a decis reluarea terapiei cu inhibitorul VEGF, cu apariţia unei fistule colovaginale complexe, documentată clinic şi imagistic. Discuţie. Tratamentul fistulei colovaginale complexe de facto presupune abolirea definitivă a anastomozei colorectale mecanice pentru a pune fistula în repaus şi a permite reducerea inflamaţiei locale, cu rezolvarea ulterioară a traiectoriei fistuloase. Colostomia definitivă este adesea percepută ca o mutilare, în special de către pacienţii tineri, care percep stoma ca o scădere a calităţii vieţii, prin impedimente de integrare socio-familială şi profesională. Concluzii. Deşi cazul nostru nu prezintă complicaţii rare ale terapiei cu bevacizumab, evidenţiază anumite probleme chirurgicale care trebuie luate în considerare la iniţierea/reluarea terapiei. Deşi există soluţii chirurgicale, în cazul pacientului nostru problema mutilării permanente se pune la un tânăr. Se ridică astfel problema dacă terapia cu bevacizumab nu ar trebui reconsiderată la anumiţi pacienţi, având în vedere că, în absenţa unui beneficiu de supravieţuire globală, disconfortul fizic şi psihic ce apare ca urmare a complicaţiilor specifice acestei terapii pot cântări mai mult decât confortul psihic indus de absenţa progresiei neoplazice. Desigur, această afirmaţie necesită studii ulterioare care ar trebui să evalueze impactul acestor complicaţii asupra pacienţilor.
Introduction
Bevacizumab therapy, indicated in the treatment of metastatic colorectal cancer, although associated with an increased progression-free survival (PFS), is frequently accompanied by certain complications that raise particular issues from a surgical point of view. Among these, gastrointestinal and complex colovaginal fistulas can raise severe problems in the surgical management of the case.
Case presentation
A 43-year-old female patient presented with transit disorders (constipation) and rectal bleeding, which started about six months priorly, being diagnosed through colonoscopy with a lower rectal tumor. The biopsy samples showed a moderately differentiated adenocarcinoma G2, with no peritumoral lymphovascular or neural invasion. The computed tomography with contrast performed for staging showed an inferior rectal parietal thickening, circumferential, with a maximum thickness of 10 mm, extending over 4.4 cm, with irregular aspect, which seemed to partially obstruct the intestinal lumen and which associated diffuse densification of perirectal fat and several perilesional adenopathies with secondary aspect, 7-8 mm in diameter. Also, the tomography described numerous pulmonary micronodules located diffusely in both lung fields with an imaging appearance suggestive for secondary lesions. The case was thus staged as cT3N1M1PUL – stage IV.
The treatment was initiated with 28 sessions of external radiotherapy associated with capecitabine. Three months after starting the therapy, the patient repeated the tomography, which revealed the dimensional progression of the secondary pulmonary lesions and the stationary appearance of the rectal lesion. The PET-CT performed confirmed the presence of metabolic activity in the lung lesions, therefore it was decided to continue chemotherapy (capecitabine and oxaliplatin associated with Avastin®). Under this treatment, the complete regression of pulmonary lesions was obtained (aspect documented by a new PET-CT). Under these conditions, the patient was referred to the department of oncological surgery, where a rectosigmoid resection was performed via the anterior route. The restauration of the digestive continuity was done by a mechanical low colorectal anastomosis accompanied by a protective ileostomy.
The postoperative evolution was hindered by the appearance, on the fifth day, of the externalization of stercoral material through the drain tubes in the pelvis in a small amount (approximately 80 ml/24 hours) – clinical aspect of anastomotic fistula. Considering the patient’s clinical condition (absence of febrile syndrome and signs of acute abdomen), the biological parameters (absence of leukocytosis), the imaging aspects (absence of pelvic collections), as well as the fact that the tubes drained the entire fistula flow, the conservative treatment of the anastomotic fistula was performed, under which the evolution was favorable, with the closure of the fistulous tract in 20 weeks, a fact confirmed by irigo-CT.
Five months after the surgery, a new PET-CT was performed which showed the recurrence of the lung lesions. The tomography performed one month later showed their numerical and dimensional progression (Figures 1 and 2). XELOX-type chemotherapy and then FOLFIRI in combination with bevacizumab were initiated. In the context of the stationary aspect of the pulmonary determinations obtained under this therapy, maintained for 10 months, the absence of rectal recurrence documented colonoscopically, as well as the option of the patient (who wanted to undergo the restauration of transit regardless of the risks presented to her), it was decided to abolish the ileostomy. The intervention took place 18 months after the rectal resection. The postoperative evolution was favorable, with the resumption of intestinal transit.
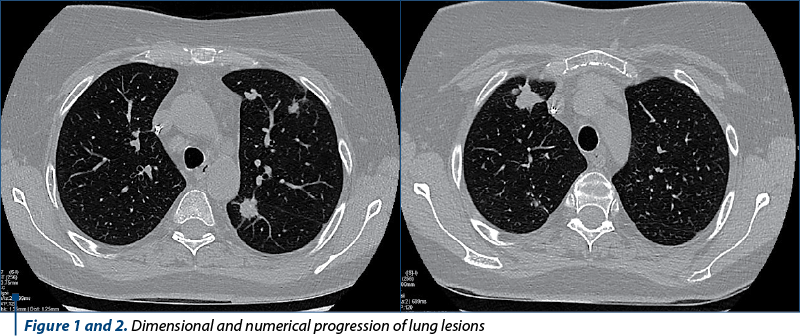
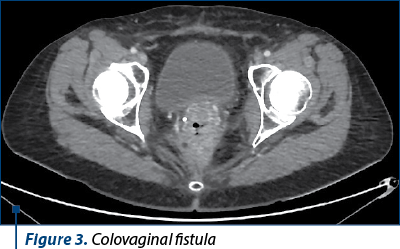
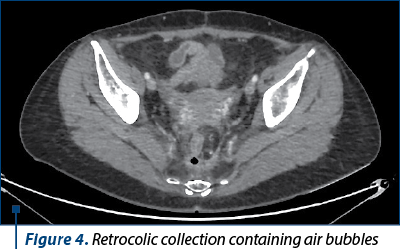
However, six months after the intervention, in the context of the maintenance therapy with bevacizumab, the patient developed a supra-anastomotic colovaginal fistula, manifested clinically by the externalization of fecal matter through the vagina and documented by imaging (on CT with irigo-CT sequences performed, it was observed both the fistulous trajectory and the result of this fistula – inhomogeneous perirectal collection containing air bubbles) (Figures 3 and 4). For the treatment of this fistula, she was addressed to the department of oncological surgery, where the patient will undergo an intervention aimed at putting the fistula at rest, allowing the reduction of the inflammatory process and the resolution per secundam of the fistulous tract. This intervention involves the abolition of the low anastomosis, the evacuation of the pelvic collection, and the performing of an external digestive diversion.
Discussion
Bevacizumab, a recombinant humanized monoclonal antibody, blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A)(1), becoming the first angiogenesis inhibitor clinically used in 2004(2). Bevacizumab was derived from a mouse monoclonal antibody immunized with the residual recombinant form of the human vascular endothelial growth factor. This form was later humanized by retaining the binding region and replacing the remainder of the protein chain with the heavy chain of a truncated human IgG1 immunoglobulin. The resulting plasmid was transferred into Chinese Hamster ovarian cells which are grown in industrial fermentation systems(3).
The development process of VEGF inhibitors in general – and of bevacizumab, in particular – began with Judah Folkman’s hypothesis in 1971 proposing that stopping angiogenesis may be useful in controlling neoplastic growth(4). Later, Napoleone Ferrara discovered a protein capable of stimulating the growth of blood vessels (VEGF)(4-6) and then demonstrated that antibodies against this protein inhibited tumor growth in mice(7), thus validating Folkman’s theory.
The neutralization of the biological activity of VEGF is done by binding to its surface receptors Flt-1 and KDR, thus causing the regression of tumor vascularization, normalizing the remaining tumor vasculature and inhibiting the formation of new vasculature, inhibiting tumor growth.
Bevacizumab indications in neoplastic diseases
In 2004, bevacizumab received the first approval in America for clinical use, in combination with standard chemotherapy, in the treatment of metastatic colorectal cancer as a first-line treatment(8), and then, in 2006, in combination with 5-fluorouracil-based therapy as a second line of treatment(9). In 2005, bevacizumab also received approval in Europe for use in metastatic colorectal cancer. There have been trials that evaluated the introduction of bevacizumab as a potentiator of chemotherapy in non-metastatic patients with colorectal carcinomas, but following two large randomized trials, it was shown that the monoclonal antibody does not increase overall survival (OS), nor does it increase the PFS. Instead, it increases the risk of adverse effects associated with the therapy(10).
In 2006, the Food and Drug Administration (FDA) approved the second clinical use of bevacizumab for the first-line therapy of advanced non-small cell lung cancer, following a randomized trial of 878 patients with stage IIIB and IV cancers, conducted by the Eastern Cooperative Oncology Group, called E4599, which showed an improvement in overall survival of two months in patients who received carboplatin and paclitaxel in combination with bevacizumab compared to those treated with chemotherapy alone (12.3 versus 10.3 months; p=0.003). Also, the study showed that, in patients with lung adenocarcinomas (which represent 85% of non-small cell lung cancers), the survival benefit was as much as four months. Although an improvement in median PFS (6.2 versus 4.5 months; p<0.001) and response rate (35% versus 15%; p<0.001) was also found in patients to whom the angiogenesis inhibitor was also associated, a significant increase in the bleeding rate was observed in these patients (4.4% versus 0.7%; p<0.001), as well as an increase of therapy-related deaths (15 therapy-related deaths in patients treated with the combination of chemotherapy and bevacizumab, including five deaths due to massive pulmonary hemorrhage)(11). In 2009, the first European clinical study on the effects of bevacizumab in non-small cell lung cancer, called AVAiL, confirmed the benefits of the progression-free survival demonstrated by the American study E4599(12). However, the European study could not prove overall survival benefit. This fact may be due to a significant bias of this study – namely, the fact that, in the AVAiL study, the use of bevacizumab as maintenance therapy was more limited than in the US study. This difference is also when comparing European and American studies performed in patients with colorectal cancer(13). In conclusion, for the therapy of advanced, unresectable, recurrent or metastatic non-small cell lung cancer, the guidelines recommend as first-line treatment platinum-based chemotherapy in combination with bevacizumab, followed by bevacizumab maintenance therapy until disease progression(14).
After the rapid approval granted in 2008 for the use of bevacizumab in the treatment of metastatic breast cancer, the FDA withdrew the authorization for this indication, due to the fact that the clinical trials conducted and completed after the approval did not show any benefit in OS or PFS, but all adverse effects of therapy were present. Initial approval had been given through the FDA’s Rapid Approval Program based on clinical trials that used an intermediate end-point (tumor shrinkage), without evaluating OS or time to progression(15). In Europe, however, bevacizumab continues to be recommended in combination with paclitaxel or capecitabine in the treatment of metastatic breast cancer.
In 2009, bevacizumab also received approvals for advanced/metastatic renal cancer (in combination with interferon alpha-2a) and glioblastoma multiforme. For both neoplastic locations, the monoclonal antibody slows down the rate of tumor growth, without having effects on overall survival(16,17).
Later, other indications for neoplastic diseases were also accepted: bevacizumab, administered in combination with carboplatin and gemcitabine/paclitaxel, is indicated for the treatment of patients diagnosed with the first recurrence of epithelial ovarian neoplasm, fallopian tube neoplasm or primary peritoneal cancers, sensitive to chemotherapy with platinum salts, who have not received previously treatment with bevacizumab or other VEGF inhibitors or targeted therapy on its receptor. It can also be administered in combination with paclitaxel and cisplatin or, alternatively, with paclitaxel and topotecan in patients who cannot be given chemotherapy with platinum salts, in patients with persistent, recurrent or metastatic cervical carcinoma.
From all of the above, it can be seen that, although bevacizumab has certain benefits of increasing the PFS, it does not increase the overall survival of treated patients.
Complications specific to therapy
Along with frequent medical complications (hypertension, congestive heart failure, arterial and venous thromboembolism, proteinuria, posterior reversible encephalopathy syndrome, ischemic and hemorrhagic stokes, neutropenia and infections, allergic reactions or eye disorders), bevacizumab therapy can cause a whole series of surgical complications (some possibly fatal), such as delayed wound healing, tumor-associated hemorrhages, pulmonary hemorrhages, aneurysms and arterial dissections, necrotizing fasciitis, or osteonecrosis of the jaw(18).
But, among the surgical complications specific to monoclonal therapy, the most formidable are the fistula complications.
Bevacizumab-treated patients have an increased risk of developing gastrointestinal or gallbladder perforations and fistulas of the digestive organs, especially in the presence of an intraabdominal inflammatory process or after radiotherapy. Gastrointestinal perforations have been reported in clinical trials at an incidence of less than 1-2% in patients treated with bevacizumab(19). In clinical trials in which bevacizumab was administered, cases of gastrointestinal fistulas (of all grades) were reported, with a high incidence in patients with colorectal neoplasms and metastatic ovarian neoplasm, but less frequently in patients with other types of neoplasia.
Patients with persistent, recurrent or metastasized cervical neoplasms also have an increased risk of the appearance of fistulas located between the vagina and any part of the digestive tract, previous radiotherapy being – in this case also – an aggravating factor(20). In a clinical trial in patients with persistent, recurrent or metastatic cervical neoplasm, the incidence of gastrointestinal-vaginal fistulas was 8.3% in bevacizumab-treated patients and 0.9% in the control group, all patients developing such complications having a history of pelvic radiotherapy(21). Patients who develop gastrointestinal-vaginal fistulas may also develop intestinal occlusions and may require surgical interventions, as well as diverting stomas.
The occurrence of these events varies in type and severity, ranging from air images in the peritoneal cavity seen on plain abdominal radiography, which can be resolved without treatment, to intestinal perforation with abdominal abscess and sometimes death. In some cases, intraabdominal inflammation coexisted, determined either by gastric ulcer disease, tumor necrosis, diverticulitis or chemotherapy-related colitis.
Death has been reported in approximately one-third of serious cases of gastrointestinal perforation, representing 0.2-1% of the total number of patients treated with bevacizumab.
Similarly, fistulas of extradigestive organs, such as tracheal or urinary, can also appear.
The surgical difficulties raised by the presented case
In our patient’s case, we did not have one, but two fistula complications associated with bevacizumab therapy. The first complication (fistula at the anastomotic level) has as probable mechanism the reduction of the healing capacity. A surgical technique defect is not possible because the fistula din not appear in the first 2-3 postoperative days. Normally, once such a complication occurs, the optimal therapeutic approach would be a surgical reintervention with the abolition of the mechanical low colorectal anastomosis and the execution of a terminal colostomy. In the context in which the patient refused a colostomy, most likely definitive (the possibilities of subsequent restoration of digestive continuity being minimal), we had to use a conservative attitude for the fistula treatment, which ensured its closure. The healing of the fistula required a long period (about four months), during which the patient required constant medical care (three weekly medical visits for repeated lavages and drainage care). This delay in healing did not allow the resumption of maintenance cytostatic therapy, this allowing the numerical and dimensional progression of the lung lesions.
With the resumption of bevacizumab therapy and the appearance, this time, of a complex supra-anastomotic colovaginal fistula, we again found ourselves in the situation of needing to put to rest the fistulous area in order to reduce the inflammatory process, thus allowing the secondary surgical treatment of the fistulous tract. We are talking about two surgical interventions, which will limit the possibilities of subsequent oncological therapy and will delay the therapy, otherwise very necessary in the context of the progressing lung lesions. The appearance of the complex fistula will require the performance of a terminal colostomy, which will lead to the devascularization of the colonic distal segment previously used for the colorectal anastomosis (the blood supply of this segment is currently achieved only at the expense of the colic arch). The resection of the distal colonic segment will, thus, be necessary. A subsequent return to transit becomes, under these conditions, illusory.
The only alternative therapeutic option would be to continue the chemotherapy for the pulmonary lesions, while accepting the difficulties of living with a colovaginal fistula, and the psychological and physical stress that comes with it.
The benefits that bevacizumab therapy could, theoretically, bring in terms of progression-free survival are countermanded by the complications that have arisen. Moreover, the overall survival of this patient may be reduced due to the impossibility of continuing cytostatic therapy in the context of the surgical interventions that become necessary. At the same time, the patient’s quality of life was diminished both from a physical point of view (the colovaginal fistula being difficult to tolerate), but also from a psychological point of view (due to the need for a definitive colostomy which the patient did not accept).
Conclusions
Although our case does not present rare complications of bevacizumab therapy, it highlights certain surgical problems that must be taken into consideration when initiating/resuming therapy. Although there are surgical solutions, in the case of our patient the problem of permanent mutilation arises in a young person. This raises the question of whether bevacizumab therapy should not be reconsidered in certain patients, considering that in the absence of an overall survival benefit, the physical and mental discomfort that occurs as a result of the specific complications of this therapy may weigh more than the mental comfort given by the absence of neoplastic progression. Of course, this statement requires further studies that should evaluate the impact of these complications on patients.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
-
Los M, Roodhart JM, Voest EE. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. The Oncologist. 2007;12(4):443–50.
-
Pollack A. FDA Approves Cancer Drug from Genentech 27 February 2004. The New York Times. Archived 24 October 2016 at the Wayback Machine. Available at: https://www.nytimes.com/2004/02/27/business/fda-approves-cancer-drug-from-genentech.html
-
European Medicines Agency. Bevacizumab Scientific Discussion, 2005. Archived 24 September 2015 at the Wayback Machine.
-
Ribatti D. Napoleone Ferrara and the saga of vascular endothelial growth factor. Endothelium. 2008;15(1):1–8.
-
Palmer AM, Stephenson FA, Williams RJ. Society for Medicines Research: 40th anniversary symposium. Drug News & Perspectives. 2007 Apr;20(3):191–6.
-
Ferrara N. Anti-angiogenic drugs to treat human disease: an interview with Napoleone Ferrara by Kristin H. Kain. Disease Models & Mechanisms. 2009;2(7–8): 324–5.
-
Ferrara N. From the discovery of vascular endothelial growth factor to the introduction of Avastin in clinical trials – an interview with Napoleone Ferrara by Domenico Ribatti. The International Journal of Developmental Biology. 2011;55(4–5): 383–8.
-
Avastin – full Prescribing Information – Genentech. Gene.com, 2011.
-
Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. The Oncologist. 2007;12(3):356–61.
-
Van Cutsem E, Lambrechts D, Prenen H, Jain RK, Carmeliet P. Lessons from the adjuvant bevacizumab trial on colon cancer: what next?. Journal of Clinical Oncology. 2011;29(1):1–4.
-
Sandler A, Gray R, Perry Michael, Brahmer J, et al. Paclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung Cancer. N Engl J Med. 2006;355:2542-2550.
-
Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 2010;21(9):1804-1809.
-
Tyagi P, Grothey A. Commentary on a Phase III Trial of Bevacizumab plus XELOX or FOLFOX4 for First-Line Treatment of Metastatic Colorectal Cancer: The NO16966 Trial. Clinical Colorectal Cancer. 2006;6(4):261-264.
-
NCCN guidelines.
-
Sasich L, Sana Rikabi S. The US FDAs withdrawal of the breast cancer indication for Avastin (bevacizumab). Saudi Pharm J. 2012 Oct;20(4):381–385.
-
Ameratunga M, Pavlakis N, Wheeler H, Grant R, Simes J, Khasraw M. Anti-angiogenic therapy for high-grade glioma. The Cochrane Database of Systematic Reviews. 2018;(11):CD008218.
-
Rini BI. Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clinical Cancer Research. 2007;13(4):1098–106.
-
https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_ro.pdf
-
Badgwell BD, Camp ER, Feig B, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008;19(3):577-582.
-
Hwang WY, Chang SJ, Kim HS, et al. Gastrointestinal/genitourinary perforation and fistula formation with or without bevacizumab in patients with previously irradiated recurrent cervical cancer: a Korean multicenter retrospective study of the Gynecologic Oncology Research Investigators Collaboration (GORILLA) group (GORILLA-1001). BMC Cancer. 2022;22(1):603.
-
Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654-1663.
Articole din ediţiile anterioare
European Commission approves Amgen and Allergan’s MVASI® (biosimilar bevacizumab) for the treatment of certain types of cancer
Amgen (NASDAQ: AMGN) and Allergan plc. (NYSE: AGN) announced in January that the European Commission (EC) has granted marketing authorization for M...