Acute myeloid leukemia (AML) is a myeloproliferative neoplasia, associated with a poor prognosis, especially when adverse genetic abnormalities are present. FLT3 mutations are particularly adverse and are found in approximately one-third of AML patients. Over the last years, a number of different FLT3 inhibitors have been studied in clinical trials. When associated with the standard treatment, overall survival and event-free survival are improved. In monotherapy, the FLT3 inhibitors are not able to maintain a durable remission. The first-generation inhibitors lack selectivity for FLT3 receptor, resulting in higher toxicity. The second-generation inhibitors have a higher potency and a higher selectivity for the FLT3 receptor, therefore lower toxicity. The patients receiving FLT3 inhibitors can lose the response, mainly due to acquired resistance.
Leucemia acută FLT3 pozitivă: exemplu de abordare personalizată
FLT3-positive acute myeloid leukemia: an example of personalized approach
First published: 23 decembrie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.53.4.2020.4045
Abstract
Rezumat
Leucemia mieloidă acută (LAM) este o neoplazie mieloproliferativă. LAM este asociată cu un prognostic nefavorabil, mai ales atunci când sunt prezente anomalii genetice clasificate în grupul de risc advers. Mutaţiile FLT3 sunt în mod particular nefavorabile şi se întâlnesc la aproximativ o treime dintre pacienţii cu LAM. În ultimii ani, numeroşi inhibitori FLT3 au fost studiaţi în trialurile clinice. În asociere cu terapia standard, supravieţuirea globală şi supravieţuirea fără evenimente adverse sunt îmbunătăţite. Inhibitorii de primă generaţie nu au selectivitate pentru receptorul FLT3, rezultând o toxicitate mai mare. Inhibitorii de a doua generaţie au o potenţă şi o selectivitate pentru receptorul FLT3 mai mari, deci şi o toxicitate mai mică. Pacienţii care primesc inhibitori FLT3 pot pierde răspunsul terapeutic, în principal din cauza rezistenţei dobândite.
Introduction
Acute myeloid leukemia (AML) is a myeloproliferative neoplasia, characterized by clonal evolution and genetic heterogeneity. In 2016, the World Health Organization (WHO) classified acute myeloid leukemia as: AML with recurrent genetic abnormalities, AML with myelodysplasia-related changes, therapy-related myeloid neoplasms, AML-NOS, myeloid sarcoma, and myeloid proliferations related to Down syndrome(1).
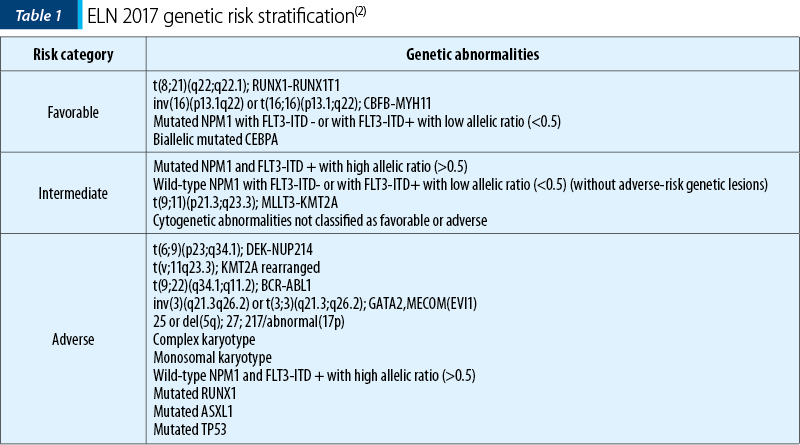
The genetic abnormalities previously described in AML have an important prognostic significance. The therapeutic decision can be influenced by their presence, and can also be targeted with specific therapy. In 2017, the European Leukemia Net (ELN) proposed a risk stratification according to the various genetic abnormalities (Table 1), also adopted by the American guideline National Comprehensive Cancer Network (NCCN)(2,3).
FLT3+ acute myeloid leukemia is a special entity in the WHO 2016 classification. FLT3 mutation is the most common and most important, considering its impact on prognosis and therapy decision.
Both ELN and NCCN guidelines recommend testing for FLT3 mutation at the diagnosis, but also at the time of progression, refractory or relapsed disease. These recommendations have high importance in administering the targeted treatment as early as indicated(2,3).
FLT3 mutation in AML
FLT3 receptor is a transmembrane receptor tyrosine kinase, coded by the FLT3 gene, located on chromosome 13q12. FLT3 is normally found in CD34+ hematopoietic stem cells, but also in a multitude of other cells(4). The binding of FLT3 ligand on the normal FLT3 receptor induces an activation of a cascade of reactions, with multiple roles in the cellular homeostasis, namely in the phospholipidic metabolism, in transcription, proliferation and apoptosis(5).
FLT3 wild-type is overexpressed in hematological neoplasms, suggesting an implication in survival and proliferation of leukemic blasts. FLT3 mutations lead to an excessive activation in AML blasts(5).
Two types of FLT3 mutations occur in AML patients, respectively FLT3-ITD (internal tandem duplications) and FLT3-TKD (tyrosine kinase domain).
FLT3-ITD is the most common FLT3 mutation, occurring in approximately 30% of cases(6). These patients should be considered for allogeneic stem cell transplantation in the first complete response (CR)(2,3). The presence of FLT3-ITD mutation is associated with a lower duration of remissions and of overall survival, and with an increased rate of relapse after allogeneic stem cell transplantation(6).
FLT3-TKD mutations have a lower incidence, only 7-10%. It is uncertain whether the presence of the mutation has a prognostic impact(6).
FLT3 inhibitors in the AML treatment
Currently, standard induction therapy is chosen based on age, fitness, comorbidities, and, more recently, genetic abnormalities. The ELN and NCCN guidelines recommend mutational screening at diagnosis for a minimum of NPM1 and FLT3 mutations, at least for the patients eligible for high-intensity induction. Also, the results should be available promptly, preferable in 48-72 hours(2).
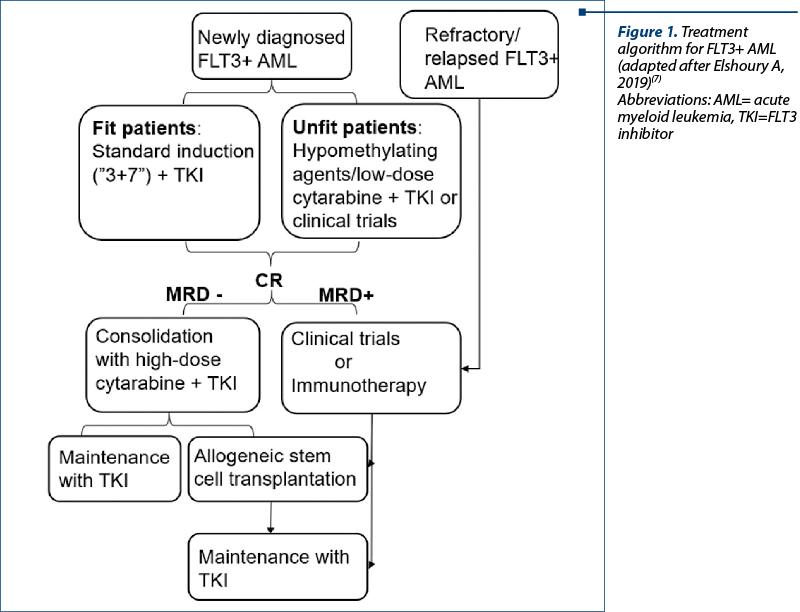
According to the current guidelines, FLT3 inhibitors are indicated from the first line. For the patients eligible for high-intensity therapy, it is used the association of FLT3 inhibitors to the standard induction chemotherapy with high-dose cytarabine and doxorubicin (“3+7” regimen). For the elderly or non-fit patients, adding FLT3 inhibitors to hypomethylating agents (azacytidine, decitabine) or low-dose cytarabine represents a valid option(2,3,7). FLT3 inhibitors are used successfully as consolidation when added to standard high-dose cytarabine, or in maintenance treatment after allogeneic stem cell transplantation (Figure 1)(3,7,8).
FLT3+ AML patients with refractory/relapsed disease have an adverse prognosis. Currently, there is no standard treatment option. FLT3 can be used in monotherapy, but their action is only on the peripheral blasts, therefore a durable response cannot be obtained(7). Second-generation inhibitors have superior responses, and the patients should be enrolled in clinical trials if possible (Figure 1)(7).
FLT3 inhibitors
There are two classifications for FLT3 inhibitors: a first one based on their specificity for FLT3 receptor, and the other based on the mechanism of action(9).
First- and second-generation inhibitors. First-generation FLT3 inhibitors are sunitinib, sorafenib, midostaurin, lestautinib, ponatinib and tandutinib. The previously mentioned have an inhibition action not only for the FLT3 receptor, but also for other tyrosine kinases. This lack of selectivity has a beneficial action in the inhibition of multiple possible leukemogenesis pathways, but the advantages come with the price of a higher toxicity(9). However, the first-generation inhibitors have only a transient effect on peripheral blast cells in monotherapy, and cannot achieve durable responses(7). Second-generation inhibitors are quizartinib, crenolanib and gilteritinib. They have a high specificity for FLT3, therefore are more potent and have lower toxicity(9).
Type I and type II inhibitors. Type I inhibitors include sunitinib, midostaurin, lestaurtinib, crenolanib and gilteritinib, while type II inhibitors include sorafenib and quizartinib. Type I inhibitors interact with the active receptor and inhibit both types of mutations, while type II inhibitors interact with the inactive conformation, therefore preventing activation. Type II inhibitors inhibit only FLT3-ITD mutation(9).
Midostaurin is the most frequently used FLT3-inhibitor in FLT3+ acute myeloid leukemia. It is a first-generation type I FLT3 inhibitor approved by the Food and Drug Administration and by the European Medicines Agency for FLT3+ AML, mastocytosis, and mast-cell leukemia(4). The RATIFY study showed a prolonged overall and event-free survival among patients with FLT3+ AML(8).
Resistance to FLT3 inhibitors therapy
Resistance to FLT3 inhibitors therapy may explain the lack of response. Both primary and acquired resistance can occur.
Primary resistance is explained by many mechanisms, such as changes in the conformation of the catalytic domain of FLT3 with a weakened affinity for the inhibitors, TKD mutation, Bcl-xL overexpression, and presence of both types of mutations (ITD and TKD)(10).
As mentioned before, the FLT3 inhibitors administered as monotherapy are unable of maintaining a durable response. Many mechanisms were described in order to explain the acquired resistance, including mutations in the FLT3 drug binding site (selection of preexisting clones or new mutation), or the acquisition of TKD mutation(7,10).
Combination regimens can be used in order to overcome the resistance to FLT3 inhibitors, with an improved response rate and a more durable remission(6).
Conclusions
FLT3-positive AML has a particularly poor prognosis. Currently, many FLT3 inhibitors are studied in clinical trials. The first-generation FLT3 inhibitors show some advantages, but the suboptimal response and the adverse reactions are significant. The second-generation FLT3 inhibitors show some progress, having better potency and specificity.
Bibliografie
-
Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
-
Dohner H, Esteyy E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129(4):424-447.
-
National Comprehensive Cancer Network. Available at: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (accessed on 1 November 2020).
-
Greer JP, Rodgers GM, Glader B, et al. Wintrobe’s Clinical Hematology: Fourteenth edition. Philadelphia, PA, Wolters Kluwer, 2019.
-
Grafone T, Palmisano M, Nicci C, et al. An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: biology and treatment. Oncol Rev. 2012;6(1):e8.
-
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutation in AML: review of current knowledge and evidence. Leuk. 2019;33:299-312.
-
Elshoury A, Przespolewski A, Baron J, Wang ES. Advancing treatment of acute myeloid leukemia: the future of FLT3 inhibitors. Expert Review of Anticancer Therapy. 2019;19(3):273-286.
-
Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. NJ Engl J Med. 2017;377:454-464.
-
Larrosa-Garcia M, Baer MR. FLT3 Inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16: 991-1001.
-
Zhou J, Chng WJ. Resistance to FLT3 inhibitors in acute myeloid leukemia: Molecular mechanisms and resensiting strategies. World J Clin Oncol. 2018;9(5):90-97.
Articole din ediţiile anterioare
Leucemie mieloidă acută M1 FAB secundară sindromului mielodisplazic cu exces de blaşti de tip 2
Leucemia acută mieloidă reprezintă o complicaţie evolutivă a sindromului mielodisplazic, mai ales în cazul mielodisplaziei cu exces de blaşti de t...
Predispoziţia la transformare din sindrom mielodisplazic în leucemie acută – prezentare de caz
Sindroamele mielodisplazice (SMD) sunt un grup de boli clonale ale celulei stem hematopoietice, caracterizate de mielodisplazie şi hematopoieză ine...
New changes in the classification of acute myeloid leukemia proposed by WHO 2022
Noua revizuire a clasificării OMS (Organizaţia Mondială a Sănătăţii) din 2022 a leucemiilor acute mieloide (LAM) are ca scop evidenţierea noilor da...