The incidence and prevalence of cancer in the older age person are increasing with the aging of the population. The management of cancer in the older patient is, to some extent, a moving target, affected by the new understanding of cancer and aging and by scientific and technological advances. The prevention and treatment of cancer in the elderly should be funded on physiologic rather than chronologic age, that is on residual life expectancy and resilience. The Comprehensive Geriatric Assessment represents at present the only instrument validated for the assessment of physiologic age. New forms of cancer treatment, including intensity modulated radiation therapy, stereotactic radiosurgery, targeted agents and immune checkpoint inhibitors, have made cancer treatment better tolerated by the elderly. Among the antidotes to chemotherapy toxicity, the “pan-protector” trilaciclib and ALRN 6924 appear most promising.
Oncologia geriatrică: principii şi perspective
Geriatric oncology: principles and perspectives
First published: 25 octombrie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.56.3.2021.5645
Abstract
Rezumat
Incidenţa şi prevalenţa cancerului la persoanele în vârstă sunt în creştere odată cu îmbătrânirea populaţiei. Gestionarea cancerului la pacientul în vârstă este într-o oarecare măsură o ţintă în mişcare, afectată de o nouă înţelegere a cancerului şi a îmbătrânirii, dar şi de progresele ştiinţifice şi tehnologice. Prevenirea şi tratamentul cancerului la vârstnici ar trebui finanţate mai degrabă în funcţie de vârsta fiziologică decât de cea cronologică, adică în ceea ce priveşte mai ales speranţa de viaţă. Evaluarea geriatrică exhaustivă reprezintă în prezent singurul instrument validat pentru evaluarea vârstei fiziologice. Noile forme de tratament pentru cancer, inclusiv radioterapia cu intensitate modulată, radiochirurgia stereotactică, terapia ţintită şi inhibitorii punctelor de control imunitar au făcut ca tratamentul cancerului să fie mai bine tolerat de către persoanele în vârstă. Printre antidoturile împotriva toxicităţii chimioterapice, trilaciclibul „panprotector” şi ALRN 6924 par a fi cei mai promiţători.
More than 50% of all cancers occur in 9.6% of the population aged 65 and older (https://www.uicc.org/what-we-do/thematic-areas-work/cancer-and-ageing#_ednref2). It is expected that the incidence and prevalence of neoplasms in older aged persons will keep increasing as long as the world population is aging. Cancer in the elderly presents new clinical and therapeutic issues which the practitioner needs to be aware of.
Clinical issues
Assessment of physiologic age
We define the physiologic age as the residual life expectancy and resilience of every person. The assessment of physiologic age, that may be quite different from the chronologic one, is the first step in the delivery of personalized care. Personalized care involves an answer to the following questions: Is the patient going to die of cancer or with cancer? Is the patient going to suffer from cancer? Is the patient able to tolerate the treatment of cancer? These questions are pertinent both to preventative and therapeutic interventions.
The assessment of physiologic age today is trusted to the comprehensive geriatric assessment (CGA)(1-2) that involves the evaluation of function in terms of basic and instrumental activities of daily living (ADL and IADL), of the presence of concomitant morbidity, polypharmacy, and of geriatric syndromes, as well as of the nutritional status, cognition, emotional status, social and economic resources. In addition to an estimate of life expectancy and of risk of therapeutic complications, the CGA may reveal conditions that interfere with the patient’s health and with the administration of antineoplastic treatment. For example, many older individuals are at risk of malnutrition that may enhance the complications of surgery, radiation and systemic cancer therapy. Others may not have access to transportation for repeated clinic and hospital visits. Others may have previously undetected diseases that need proper treatment or functional limitations that may benefit from pre-habilitation.
Many practitioners complained that a full CGA may represent an excessive investment of time because in practice we need a 4-6 full assessment. Of these, the G8, the VES 13 and and the SAOP 2 have gained widespread acceptance(3).
At least three instruments have been developed to estimate life expectancy from the CGA(4-6) and several studies have associated the geriatric assessment with the risk of hematological and non-hematological complications of chemotherapy, and with the risk of early mortality and functional decline during cancer treatment(7,8).
A number of biological markers of aging, including the inflammatory index, the genomic clock and the circulating vitamin D level, may also predict the mortality risk and, as such, they may be utilized in the assessment of physiologic age(9). At present, it is not clear whether these assays will substantially improve the clinical estimate of physiologic age.
The CGA is multidimensional and involves socioeconomic, social and medical issues in addition to the medical ones. Any intervention based on the CGA may require a team of different experts.
The management team
The management of cancer may involve interweaving surgical, radiation and systemic treatment and, therefore, it is multidisciplinary. In the case of the older patients, the team should include a social worker, a dietitian, a pharmacist and a physical therapist, if anyway possible(10).
The role of the social worker is multifaceted and includes the assessment of the living condition, and in particular of the competence of the home caregiver, the provision of economic help and transportation, if necessary, the evaluation of the cognitive status and the emotional support of the older patient and his/her caregiver(s). The importance of the home caregiver cannot be overstated. An effective caregiver represents the most important ally of the practitioner. She/he may enable the compliance with the treatment and maintain the patient’s function and welfare by assuring exercise, nutrition, transportation to the clinics, timely management of emergencies and emotional support of the patient. It behooves the practitioner to prevent the caregiver burn-out that may jeopardize the patient’s treatment and the caregiver’s health. For this goal, the practitioner should support the caregiver with instructions, recommendations, encouragement and praise. The dietitian assesses the nutritional status, the nutritional risk, the nutritional access and the need for nutritional support and supplements. Approximately 30% of individuals aged 65 and older are at nutritional risk and the percentage increases with age and with the diagnosis of cancer(11). The pharmacist is responsible for the management of polypharmacy(11), that becomes more and more prevalent with the advancing age. This includes the risk of drug interactions, the elimination of dangerous or redundant medications and the addition of new drugs if indicated.
It is almost universal that, after the age of 70 years old, the polypharmacy may cause drug interactions, drug-related illnesses and financial difficulties. The use of electronic health record has facilitated the communication among different specialists and mitigated the risk of polypharmacy. Still, a periodic review of a patient’s prescription and nonprescription drugs by a pharmacist is desirable. This may allow to identify drugs that are best to be avoided in older individuals, drug redundancy, risk of drug interactions and also the inadequate management of some of the patient’s conditions. The management of chronic conditions may be particularly challenging in older patients with cancer. For example, is the tight control of diabetes, hypertension or cholesterol indicated in individuals with limited life expectancy or is it better forgone to ameliorate the risks of polypharmacy?
The physical therapist may establish whether exercise may improve the function of older patients and in particular if they are at risk for falls(13). Fall avoidance is important to prevent injury, such as bone fractures, that may compromise the patient’s welfare and jeopardize the timely treatment of cancer. In addition, fear of falling may unnecessarily limit a person’s mobility.
The summary of the team finding and recommendations should ideally be formulated at the end of the visit. Alternatively, it may be finalized at a regularly scheduled board meeting when all the new patient’s conditions are reviewed.
Coordination of care
In most cases, older individuals with multiple morbidities are managed by different specialists. Coordination of care is necessary to prevent conflictual prescriptions, redundancy, and to relieve as much as possible the burden of multiple clinic visits. At the very minimum, each practitioner should be aware of the geriatric assessment and of the functional, social and emotional needs of the patients, as well as of his/her pharmacy. Ideally, each older individual should have a primary care provider capable to advise him/her when the recommendations of different specialist are conflictual. Electronic health records may facilitate coordination of care and telemedicine my help reducing the burden of in person visit.
Management of cancer in the elderly
Cancer prevention
Primary prevention of cancer through lifestyle modifications (quitting smoking, exercise, diet) is recommended at any age. Indeed, it is important to underline that primary prevention may be especially effective in older individuals, as age is associated with an increased susceptibility to environmental carcinogens. Chemoprevention of cancer is promising but so far it has limited applications. Vaccines promise to be very effective in the prevention of HPV-related cancer of the lower genital tract in women, but their effectiveness has been demonstrated only when they are administered to pre-puberal children.
In this section, we will discuss the secondary prevention of cancer or the prevention of cancer death through early detection in asymptomatic individuals(14). Age is associated with increased incidence and prevalence of common cancers and, consequently, the screening may be more productive in older than in younger people. At the meantime, age is associated with an increased risk of competitive causes of death and the benefits of cancer screening on survival may be lessened in older individuals. Other age-related factors that may contraindicate the screening include the risk of overdiagnosis and overtreatment, that is the risk to diagnose cancer that may never affect the survival and the welfare of the patient and to submit the patients to unnecessary, risky and costly interventions.
The conditions that justify the secondary prevention of cancer include a population at high risk, the availability of minimally invasive, affordable and highly accurate screening intervention, and patients whose mortality risk may be reduced by the early treatment of cancer. The definitive proof of effectiveness of secondary prevention is the demonstration in randomized controlled trials that the screening has reduced the cancer-related mortality.
In Table 1, there are listed the interventions that were proven effective(14).
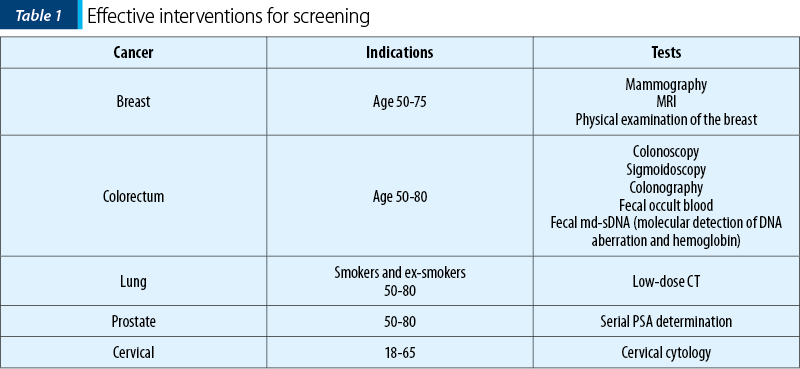
Without getting into the merit of each intervention, some general observations must be mentioned.
The benefits of screening beyond a certain age were not demonstrated, because randomized prospective studies included only younger patients. It is not legitimate to conclude from these results that older patients did not benefit from screening. Indeed, a number of retrospective analyses of cancer registries showed that women with minimal or moderate comorbidity experienced a decrease risk of mortality when they underwent regular mammograms at least up to the age of 85 years old(15,16).
Most studies included a limited number of screening sessions at different time intervals. Even the most conservative screening recommendations in the USA may have more frequent screening sessions.
Randomized controlled studies of screening may take a long time to conclude and, thus, they may not reflect the demographic, epidemiologic, and technological changes. Perhaps the case of prostate cancer offers the best example of how these changes may affect cancer screening.
Two large randomized controlled studies of prostate cancer screening with serial PSA determination were recently reanalyzed according to the Cancer Intervention and Surveillance Modeling Network of the National Cancer Institute (CISNET). According to this analysis, both studies showed a reduction in the prostate cancer-related mortality of 25-32% at 10 years. Based on this result, the screening for prostate cancer may be recommended in men aged 55-70 years old. However, a number of changes have occurred since the initiation of these studies. First of all, the percentage of men aged 85 and older has dramatically increased. Secondly, it became clear that age is a risk factor for developing lethal forms of prostate cancer. Many of these cancer-related deaths in individuals 85 and older might be prevented if the upper age of screening were increased to 75 or 80. At the meantime, some of the risks related to the diagnosis and treatment of prostate cancer have been minimized(18). When the studies were conducted, the diagnosis was established by ultrasound directed transrectal biopsy which was associated with a 2% risk of hospitalization for sepsis. Today, the multiparametric MRI allows the performance of less biopsies targeted on high-risk areas. The use of intensity modulated radiotherapy or radiosurgery for local treatment in lieu of radical prostatectomy or external beam irradiation appears well tolerated by individuals of any age.
The case of cervical cancer provides another example of how current screening recommendation may be obsolete. In the last 20 years, it has become clear that age is associated with an increased risk of cervical cancer and of cervical cancer-related mortality(19-20). Many of these deaths might be preventable by extending the screening beyond the age of 65 for women who have not undergone hysterectomy.
When multiple screening interventions are available, the common sense should direct the choice. Although the benefits of clinical examination of the breast by a professional were never established in randomized controlled trials, this is a simple examination that could be conducted in each doctor office at each clinic visits and should be recommended. The preparation for colonoscopy or colonography may be very uncomfortable for older patients and may cause dehydration. Fecal md-sDNA is very simple and can be performed on voided stools from the patient residence(21). The convenience may make it preferable for older individuals with limited mobility and access to transportation.
A reasonable question concern is represented by which older patients should be screened. In our opinion, the decision should be based on physiologic rather than chronologic age(22). Some form of screening should be offered to individuals with a life expectancy of at least five years, since the initial benefits of screening may be seen in the first five years since the initiation of screening.
The most promising investigations related to cancer screening in asymptomatic individuals concern the study of circulating free DNA that may represent the earliest marker of multiple neoplasms(23). This form of “pan-screening” may prove particularly appropriate for older individuals who are susceptible to develop multiple malignancies(24).
Cancer treatment
General principles
Twenty years ago, I proposed an algorithm for the treatment of cancer in the elderly, based on life expectancy and resilience, assessed with the CGA25 (Figure 1).
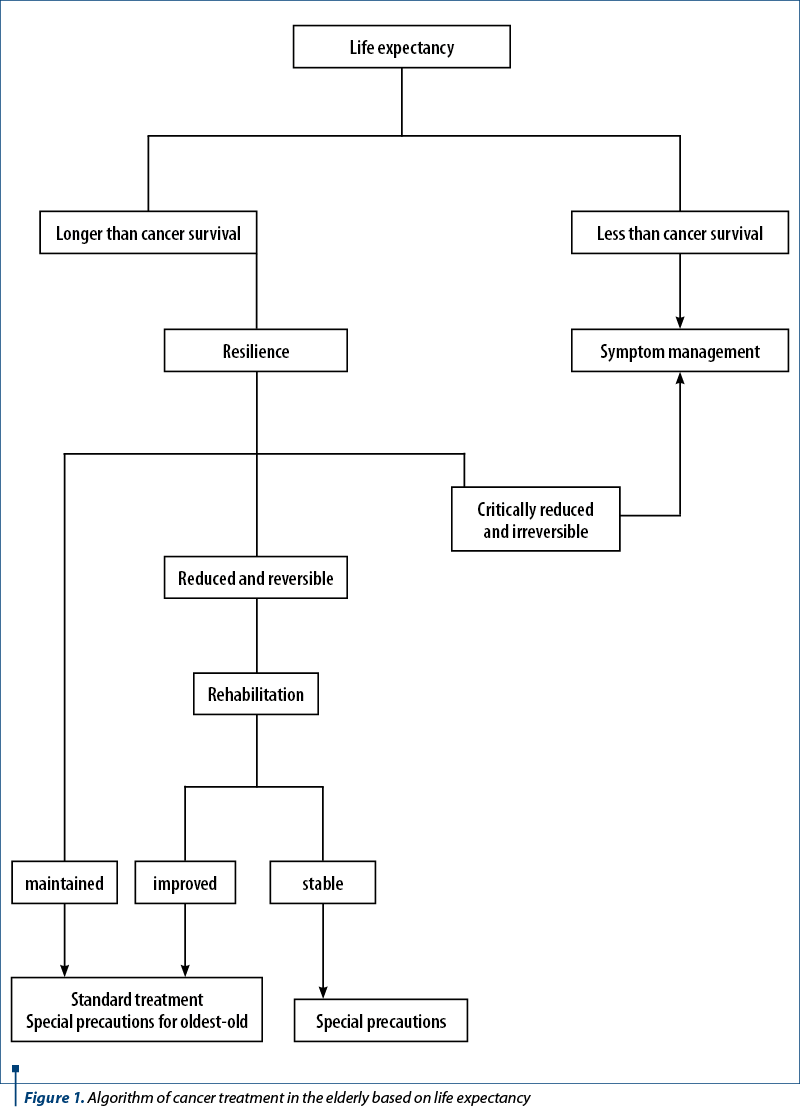
In older individuals, the treatment-related risks may overcome the benefits due to a reduction of life expectancy and treatment tolerance. As a general principle, physiologic age should prevail over chronologic age in management-related decisions. At the meantime, in the absence of information on the oldest-old, it may be prudent to assume that resilience may be critically reduced even in the presence of normal function and in the absence of severe comorbidities beyond a certain age. For this reason, it was recommended that, in the oldest-old one, it may proceed with some caution. For example, in an oldest-old patient with large cell lymphoma, it may be prudent to reduce by 25% the dose of doxorubicin during the first administration of CHOP and to escalate the dose with the second cycle of chemotherapy if no adverse events were observed with the first cycle. Currently, individuals aged 85 and older are considered oldest-old, but this number may change as more information becomes available(26).
In addition to cure, symptom palliation and prolongation of survival – the goals of cancer treatment in the older person – should include prolongation of active life expectancy(27), as loss of functional independence is associated with a major deterioration of quality of life in older individuals. For this reason, it is desirable to obtain a so-called “value history”. This should assess what kind of advanced activities of daily living are important to the patient and how many of these activities the patient is ready to sacrifice in order to obtaine a cure or a prolongation of survival(28).
In the following, there are briefly reviewed the principles of localized and systemic cancer treatment in older patients.
Locoregional treatment
Surgery
Age does not appear associated with an increased mortality for elective surgery, while older patients may experience a more prolonged postoperative hospitalization and rehabilitation(29). Age is a risk factor for mortality and other complications of emergency surgery(30). Regular screening of older patients for colorectal cancer may prevent emergency interventions for intestinal obstructions(29). The preoperative evaluation with a CGA is of paramount importance in order to identify patients at an increased risk due to reduced resilience and to institute preoperative interventions that may ameliorate the prognosis. These may include nutritional support, optimal management of comorbidity and prehabilitation. The surgical prognosis has improved in older patients during the past 20 years, thanks to safer anesthesia and the institution of minimally invasive surgery(31).
Radiation therapy
The acute complications of external beam irradiation – including mucositis, dehydration and myelosuppression – increase with age and a CGA allows to identify the patients at risk who may benefit from less aggressive schedules of treatment(32).
It is important to underline a number of advances in radiation oncology that may be particularly beneficial to the elderly, as they reduce the exposure of healthy tissues to radiation. These include brachytherapy, intensity modulated radiation therapy (IMRT) and stereotactic ablative radiation therapy (SART), also referred to as stereotactic radiosurgery (SRS). SRS obtains results comparable to those of surgery in brain tumors and in the early stages of lung cancer; IMRT of the prostate is comparable to radical prostatectomy, at least for the first 10 years following treatment.
Locoregional treatment of metastatic disease
The concept of oligometastatic disease is germane to the management of older patients. The oligometastatic concept holds that, in patients with low number of metastases and slow tumor progression, the locoregional management of the metastasis may prolong the survival, without the use of cytotoxic chemotherapy(33). A number or recent studies have shown the benefits of this approach(34-35). In addition to IMRT and SRS, metastases may be treated with a number of percutaneous ablation techniques, that include radiofrequency, cryoablation, microwave ablation and irreversible electroporation(36).
Systemic cancer therapy
Cytotoxic chemotherapy
Cytotoxic chemotherapy is still the mainstay of the treatment of metastatic cancer. The risk of complications of chemotherapy – including myelosuppression, mucositis, cardiomyopathy, peripheral and central neuropathy – increases with age, due to a combination of factors(37). Of these, certainly the most important is the reduction in functional reserve of organ systems that are targets of toxicity (pharmacodynamic effect). A number of pharmacokinetics effects (decreased renal excretion and hepatic metabolisms, decreased volume of distribution of water-soluble agents and increased risk of drug interactions from polypharmacy) may also contribute to the enhanced toxicity of chemotherapy.
A number of provisions may ameliorate the risk of chemotherapy in older individuals. These include, first of all, patients selection based on CGA, reduction of the doses of chemotherapy in the oldest-old and in all patients with decreased resilience, and the use of antidotes to toxicity, such as the myelopoietic growth factor to prevent neutropenia and of cryotherapy and highly saturated salt solutions to prevent mucositis. Of special interest is the development of new drugs that promise to prevent the multiple complications of chemotherapy, by inhibiting the proliferation of normal cells during the chemotherapy administration. Trilaciclib is an inhibitor of CDK4/6 and was approved for small lung cancer patients receiving topotecan(38). ALRN 6926 is an inhibitor of P53 and is being tested in different P53 wild tumors(39). These compounds hold many important promises in older patients. First, they may prevent anemia and thrombocytopenia in addition to neutropenia and may bypass the suppressive action of myelopoietic growth factors over other hemopoietic lines (stem cell competition). The avoidance of anemia that cannot be obtained with the current limitations on the use of erythropoietic growth factors may prevent fatigue that is a harbinger of functional decline and death in older individuals. Secondly, they may elude the development of myelopoietic malignancies associated with growth factors. Thirdly, they may prevent diarrhea and mucositis which in older patients are a cause of severe dehydration.
Hormonal treatment
Hormones have provided the first systemic treatment of cancer and are still the mainstay treatment of hormone receptor-rich breast cancer and of prostate cancer. The main complications of aromatase inhibitors in breast cancer and of androgen deprivation in prostate cancer consist in osteoporosis and bone fractures. In these patients, it is advisable to supplement the diet with calcium and vitamin D, to enact fall prevention, and to add to the treatment bisphosphonates or denosumab. Aromatase inhibitors may cause disabling arthralgia, and androgen deprivation causes fatigue, anemia, cardiovascular complications and, possibly, cognitive decline.
Targeted treatment
Medications aiming at a specific molecular target include different classes of drugs, such as monoclonal antibodies and small molecule inhibitors of critical enzymatic reactions(40). In general, targeted treatment is better tolerated than cytotoxic chemotherapy by most patients. The risk of some specific complications may be increased in older patients. This includes the risk of cardiotoxicity from trastuzumab, of thrombosis from angiogenesis inhibitors and of diarrhea and peripheral neuropathy from different compounds.
Immune checkpoint inhibitors
Immune checkpoint inhibitors had undoubtedly a major effect on survival of cancer patients that may be comparable to the effects of cytotoxic chemotherapy(41,41). Age does not seem to affect the effectiveness or the toxicity of these agents. Immune checkpoint inhibitors appear effective and well tolerated also in patients with limited resilience and severe comorbidities.
New perspectives on the systemic treatment of cancer
In Figure 1, I had recommended that patients with life expectancy shorter than cancer-related survival or patients with reduced treatment tolerance receive only palliative management. This recommendation was well supported at a time when the mainstay systemic treatment of cancer was systemic chemotherapy. The advent of immune checkpoint inhibitors and, to a lesser extent, of targeted therapy invites to review this recommendation. In particular, one has to ask three questions: May a remission of cancer improve the performance status and the overall life expectancy of the patient? Do targeted therapy or immunotherapy represent the most effective management of cancer-related symptoms? May we justify from an ethical standpoint to withhold a treatment that may benefit the patients with minimal toxicity because it is costly and, in our estimate, unlikely to help?
There may be different ways to approach these questions. In my opinion, the most reasonable course of action appears to administer whatever treatment that promise to be beneficial as long as it does not risk to jeopardize the patient’s quality of life. After a reasonable trial time (six to twelve weeks), if no benefits are observed in terms of cancer response or improvement in quality of life, the treatment should be discontinued as futile.
Conclusions
With the aging of the population, cancer in the elderly is becoming an increasingly common problem.
In planning the prevention and the treatment of cancer in the elderly, the practitioner should consider the physiologic age of the patients, that is the life expectancy and the residual resilience. The most reliable way to assess the physiologic age is a CGA that allows the practitioner to discover conditions that may interfere with cancer treatment, such as poorly controlled comorbidity, nutritional risk, inadequate caregiver, or limited access to care.
Recent developments have made some cancer treatment also available for individuals with limited resilience. These include minimally invasive surgery, percutaneous ablation of neoplastic lesions, IMRT, SRS, targeted systemic therapy and immune checkpoint inhibitors.
Conflict of interests: The author declares no conflict of interests.
Bibliografie
-
Lichtman SM, Cohen HJ, Muss H, et al. From Assessment to Implementation and Beyond in Cancer and Aging Research. J Clin Oncol. 2021 Jul 1;39(19):2217-2225. doi: 10.1200/JCO.21.00317.
-
Nightingale G, Battisti NML, Loh KP, et al. Perspectives on functional status in older adults with cancer: An interprofessional report from the International Society of Geriatric Oncology (SIOG) nursing and allied health interest group and young SIOG. J Geriatr Oncol. 2021 May;12(4):658-665. doi: 10.1016/j.jgo.2020.10.018.
-
Garcia MV, Agar MR, Soo WK, et al. Screening Tools for Identifying Older Adults with Cancer Who May Benefit From a Geriatric Assessment: A Systematic Review. JAMA Oncol. 2021 Apr 1;7(4):616-627. doi: 10.1001/jamaoncol.2020.6736.
-
Cruz M, Covinsky K, Widera EW, et al. Predicting 10-year mortality for older adults. JAMA. 2013 Mar 6;309(9):874-6. doi: 10.1001/jama.2013.1184.
-
Schonberg MA, Li V, Marcantonio ER, Davis RB, et al. Predicting Mortality up to 14 Years Among Community-Dwelling Adults Aged 65 and Older. J Am Geriatr Soc. 2017 Jun;65(6):1310-1315. doi: 10.1111/jgs.14805.
-
Timmermans EJ, van Schoor NM, van Zutphen EM, et al. The Longitudinal Aging Study Amsterdam: cohort update 2019 and additional data collections. Eur J Epidemiol. 2020 Jan;35(1):61-74. doi: 10.1007/s10654-019-00541-2. Epub 2019 Jul 25.
-
Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults with Cancer. J Clin Oncol. 2016 Jul 10;34(20):2366-71. doi: 10.1200/JCO.2015.65.4327.
-
Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012 Jul 1;118(13):3377-86. doi: 10.1002/cncr.26646.
-
Sedrak MS, Gilmore NJ, Carroll JE, et al. Measuring Biologic Resilience in Older Cancer Survivors. J Clin Oncol. 2021 Jul 1;39(19):2079-2089. doi: 10.1200/JCO.21.00245.
-
Chapman AE, Swartz K, Schoppe J, Arenson C. Development of a comprehensive multidisciplinary geriatric oncology center, the Thomas Jefferson University Experience. J Geriatr Oncol. 2014 Apr;5(2):164-70. doi: 10.1016/j.jgo.2014.01.003.
-
Kaiser MJ, Bauer JM, Rämsch C, et al. Mini Nutritional Assessment International Group. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010 Sep;58(9):1734-8. doi: 10.1111/j.1532-5415.2010.03016.x.
-
Muth C, Blom JW, Smith SM, et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. J Intern Med. 2019 Mar;285(3):272-288. doi: 10.1111/joim.12842. Epub 2018 Dec 10. Erratum in: J Intern Med. 2019 Oct;286(4):487. PMID: 30357955.
-
Magnuson A, Sattar S, Nightingale G, Saracino R, Skonecki E, Trevino KM. A Practical Guide to Geriatric Syndromes in Older Adults with Cancer: A Focus on Falls, Cognition, Polypharmacy, and Depression. Am Soc Clin Oncol Educ Book. 2019 Jan;39:e96-e109. doi: 10.1200/EDBK_237641.
-
Balducci L. Cancer Prevention in the Older Individual. Semin Oncol Nurs. 2016 Aug;32(3):314-24. doi: 10.1016/j.soncn.2016.05.011.
-
McCarthy EP, Burns RB, Freund KM. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000 Oct;48(10):1226-33. doi: 10.1111/j.1532-5415.2000.tb02595.x.
-
McPherson CP, Swenson KK, Lee MW. The effects of mammographic detection and comorbidity on the survival of older women with breast cancer. J Am Geriatr Soc. 2002 Jun;50(6):1061-8. doi: 10.1046/j.1532-5415.2002.50261.x.
-
Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the Effects of Screening on Prostate Cancer Mortality in the ERSPC and PLCO Trials. Ann Intern Med. 2017 Oct 3;167(7):449-455. doi: 10.7326/M16-2586.
-
Catalona WJ. Prostate Cancer Screening. Med Clin North Am. 2018 Mar;102(2):199-214. doi: 10.1016/j.mcna.2017.11.001.
-
Eun TJ, Perkins RB. Screening for Cervical Cancer. Med Clin North Am. 2020 Nov;104(6):1063-1078. doi: 10.1016/j.mcna.2020.08.006.
-
White MC, Shoemaker ML, Benard VB. Cervical Cancer Screening and Incidence by Age: Unmet Needs Near and After the Stopping Age for Screening. Am J Prev Med. 2017 Sep;53(3):392-395. doi: 10.1016/j.amepre.2017.02.024.
-
Carethers JM. Fecal DNA Testing for Colorectal Cancer Screening. Annu Rev Med. 2020 Jan 27;71:59-69. doi: 10.1146/annurev-med-103018-123125.
-
Kotwal AA, Walter LC. Cancer Screening in Older Adults: Individualized Decision-Making and Communication Strategies. Med Clin North Am. 2020 Nov;104(6):989-1006. doi: 10.1016/j.mcna.2020.08.002.
-
Tanos R, Tosato G, Otandault A,et al. Machine Learning-Assisted Evaluation of Circulating DNA Quantitative Analysis for Cancer Screening. Adv Sci (Weinh). 2020 Jul 29;7(18):2000486. doi: 10.1002/advs.202000486.
-
Luciani A, Balducci L. Multiple primary malignancies. Semin Oncol. 2004 Apr;31(2):264-73. doi: 10.1053/j.seminoncol.2003.12.035.
-
Balducci L. Geriatric oncology. Crit Rev Oncol Hematol. 2003 Jun;46(3):211-20. doi: 10.1016/s1040-8428(03)00020-9.
-
Cook E, Gershman S, Knowlton R. Cancers Among the Oldest Old in Massachusetts from 2004-2014. J Registry Manag. 2018 Spring;45(1):21-27.
-
Jia H, Lubetkin EI. Life expectancy and active life expectancy by disability status in older U.S. adults. PLoS One. 2020 Sep 25;15(9):e0238890. doi: 10.1371/journal.pone.0238890.
-
Dias EN, da Silva JV, Pais-Ribeiro JL,et al. Validation of the advanced activities of daily living scale. Geriatr Nurs. 2019 Jan-Feb;40(1):7-12. doi: 10.1016/j.gerinurse.2018.05.008.
-
Montroni I, Rostoft S, Spinelli A, et al. SIOG surgical task force/ESSO GOSAFE study group. GOSAFE - Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery: early analysis on 977 patients. J Geriatr Oncol. 2020 Mar;11(2):244-255. doi: 10.1016/j.jgo.2019.06.017.
-
Torrance AD, Powell SL, Griffiths EA. Emergency surgery in the elderly: challenges and solutions. Open Access Emerg Med. 2015 Sep 8;7:55-68. doi: 10.2147/OAEM.S68324.
-
Bettelli G. Preoperative evaluation of the elderly surgical patient and anesthesia challenges in the XXI century. Aging Clin Exp Res. 2018 Mar;30(3):229-235. doi: 10.1007/s40520-018-0896-y.
-
Chang S, Goldstein NE, Dharmarajan KV. Managing an Older Adult with Cancer: Considerations for Radiation Oncologists. Biomed Res Int. 2017;2017:1695101. doi: 10.1155/2017/1695101.
-
Loi M, Alifano M, Scorsetti M, et al. Judging a Fish by Its Ability to Climb a Tree? A Call for Novel Endpoints in the Appraisal of Ablative Local Treatments of Oligometastatic Cancer. Oncologist. 2021 Jun;26(6):e1085-e1086. doi: 10.1002/onco.13747.
-
Macchia G, Lazzari R, Colombo N, et al. A Large, Multicenter, Retrospective Study on Efficacy and Safety of Stereotactic Body Radiotherapy (SBRT) in Oligometastatic Ovarian Cancer (MITO RT1 Study): A Collaboration of MITO, AIRO GYN, and MaNGO Groups. Oncologist. 2020 Feb;25(2):e311-e320. doi: 10.1634/theoncologist.2019-0309.
-
Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020 Sep 1;38(25):2830-2838. doi: 10.1200/JCO.20.00818.
-
Arellano RS. What’s New in Percutaneous Ablative Strategies for Hepatocellular Carcinoma and Colorectal Hepatic Metastases? 2020 Update. Curr Oncol Rep. 2020 Jul 28;22(10):105. doi: 10.1007/s11912-020-00967-y.
-
Hurria A, Wildes T, Blair SL, et al. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Net. 2014 Jan;12(1):82-126. doi: 10.6004/jnccn.2014.0009.
-
Weiss JM, Csoszi T, Maglakelidze M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019 Oct 1;30(10):1613-1621. doi: 10.1093/annonc/mdz278.
-
Zhou X, Singh M, Sanz Santos G, et al. Pharmacological activation of p53 triggers viral mimicry response thereby abolishing tumor immune evasion and promoting anti-tumor immunity. Cancer Discov. 2021 Jul 6:candisc.1741.2020. doi: 10.1158/2159-8290.CD-20-1741. PMID: 34230007.
-
Daste A, Chakiba C, Domblides C, et al. Targeted therapy and elderly people: A review. Eur J Cancer. 2016 Dec;69:199-215. doi: 10.1016/j.ejca.2016.10.005.
Articole din ediţiile anterioare
Importanţa screeningului nutriţional la pacientul chirurgical oncologic
Potrivit unui studiu realizat la Universitatea Johns Hopkins, din SUA, până la 50% dintre pacienţi au deficienţe nutriţionale la internarea în spi...