Preoperative chemoradiotherapy (p-CRT) is a widely used technique for treating locally advanced rectal cancer, esophageal cancer, gastric cancer and other cancers, with different success rates and different patterns of response. The medical literature has long sought to establish an accurate predictive model for rectal cancer’s tumor response to neoadjuvant treatment. Our current study (which has only preliminary results) aims at investigating the correlation between several immunohistochemistry markers and the tissue response to preoperative radiochemotherapy in rectal cancer. The research examined a batch of 29 patients diagnosed with rectal cancer (adenocarcinoma) who had received radiochemotherapy prior to the surgical intervention and compared the pathology result from the biopsy at the moment of the diagnostic (RIB) with the pathology result from the surgical specimen (RIC). Out of all the markers studied, we will further discuss the literature context and the results concerning the markers that exhibited a statistically significant variation in response to radiochemotherapy as a neoadjuvant treatment (p53, MMP-1, MMP-13, TIMP-1, TIMP-3). The conclusion is that further prospective studied associating clinical outcome with variations in these parameters would be more reliable and could even lead to the implementation of a prediction model of the tissue response to neoadjuvant treatment (radiochemotherapy) in rectal cancer.
Preliminary results of markers as predictors for the tissue response to radiochemotherapy in rectal cancer
Rezultate preliminare ale markerilor ca predictori ai răspunsului tisular la radiochimioterapie în cancerul rectal
First published: 25 martie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.58.1.2022.6223
Abstract
Rezumat
Radiochimioterapia preoperatorie (p-CRT) este o tehnică utilizată pe scară largă pentru tratarea cancerului rectal local avansat, a cancerului esofagian, cancerului gastric şi a altor tipuri de cancer, cu rate de succes diferite şi modele diferite de răspuns la tratamentul neoadjuvant. Literatura medicală a căutat de mult timp să stabilească un model predictiv precis pentru răspunsul tumoral al cancerului rectal la tratamentul radiochimioterapic. Acest studiu (care are doar rezultate preliminare) îşi propune să investigheze corelaţia dintre mai mulţi markeri imunohistochimici şi răspunsul tisular la radiochimioterapia preoperatorie în cancerul rectal. Cercetarea a examinat un lot de 29 de pacienţi diagnosticaţi cu tumori maligne rectale (adenocarcinoame) care au efectuat radiochimioterapie înainte de intervenţia chirurgicală şi a comparat rezultatul de anatomie patologică din biopsie la momentul diagnosticului (RIB) cu rezultatul histopatologic din specimenul chirurgical (RIC). Dintre toţi markerii studiaţi, vom discuta în continuare baza teoretică şi rezultatele în cazul markerilor care au prezentat o variaţie semnificativă statistic în răspunsul la tratamentul neoadjuvant (p53, MMP-1, MMP-13, TIMP-1, TIMP-3). Concluzia este că studii prospective ulterioare, care ar putea asocia rezultatul clinic cu variaţiile acestor parametri, ar fi mai fiabile şi ar putea chiar conduce la implementarea unui model de predicţie a răspunsului tisular la tratamentul neoadjuvant (radiochimioterapie) în cancerul rectal.
Preoperative chemoradiotherapy (p-CRT) has been widely used to treat locally advanced rectal cancer, esophageal cancer, gastric cancer and other cancers. In rectal cancer, approximately 60% of tumors exhibit tumor regression and T and N downstaging. Additionally, a pathologic complete response (p-CR), defined as the sterilization of all tumor cells, results in an excellent prognosis and occurs in approximately 10% to 30% of cases(1). However, the chemoradiation therapy has a number of serious adverse effects, and some patients exhibit a poor or nonresponsive pattern.
Medical literature has long been trying to establish an accurate prediction model for the tumor response to preoperative radiochemotherapy in rectal cancer. For that purpose, many aspects have been studied, from various clinical settings(2,3), imagery parameters(4), histology(5), immunohistochemistry(6), cytology(7) and genetics(8). Our present study (with only preliminary results, so far) further looks into attempts to correlate several markers of immunohistochemistry to the tissue response to preoperative radiochemotherapy in rectal cancer.
Our research focused on a batch formed out of 29 patients diagnosed with rectal cancer (adenocarcinoma) which had received radiochemotherapy before the surgical intervention, under the form of a neoadjuvant treatment. The post-radiotherapy changes of the following immunohistochemical markers were analyzed: MMP1, MMP2, MMP3, MMP9, MMP11, MMP13, p63, TIMP1, TIMP2, TIMP3, BCL-2, Cycline D1, Vimentin, CEA, E-cadherin, p53, CK-7, CK-20. Out of those, we will further present in this article the literature context and the measured results only regarding the markers presenting with a statistically significant variation with radiochemotherapy.
P53
It is a tumor suppressor gene that can have a mutation rate of up to 50%, leading towards severe consequences, such as damage in the response to cellular stress and DNA damage. From an anatomical and pathological point of view, the mutation at the p53 level transforms the late adenoma into adenocarcinoma.
The p53 protein is the central monitor of the cellular lesion potential and can be activated by anoxia, inadequate signaling of oncogenes, and the activity of genes involved in cell cycle arrest, DNA repair, cell aging and apoptosis(9).
Research presented by Yang et al.(10) showed that recurrent mutations in TP53 confer selective advantages on cancer cells and render them resistant to CRT. Another study by Huang et al.(11) took into consideration the fact that the radiosensitivity of tumors is affected by the expression of p53 binding protein 1 (53BP1) and the density of T lymphocytes infiltration. The conclusion was that pretreatment levels of 53BP1 and an immunoscore based on the density of CD3+/CD8+ T cells in tumor tissues are both effective predictors of CRT response, with 53BP1 having a greater impact on prognosis.
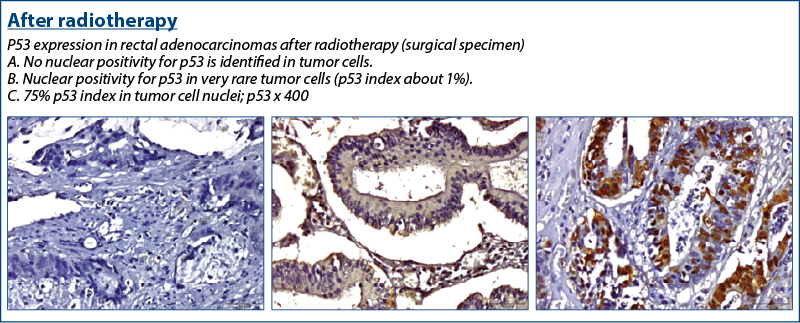
A high p53 expression in the pretreatment biopsy was associated with an incomplete response in surgical resection specimens after neoadjuvant treatment, as found by el Otmani et al.(12)
In our group of patients, the following results were found regarding p53 and its variation with radiochemotherapy.
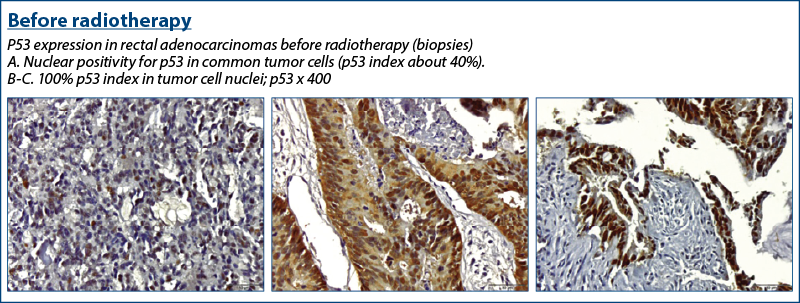
Metalloproteinases (MMPs)
MMPs are a group of zinc-dependent endopeptidases that promote tumor dissemination and metastasis by degrading the extracellular matrix (ECM) and basal membrane.
There are over 20 MMPs classified as collagenases (MMP-1, -8, -13, and -18), gelatinases (MMP-2 and MMP-9), stromelysins (MMP-3, -7, -10, -11, -26, -27), elastases (MMP-12), membrane type-specific MMPs (MMP-14, -15, -16, -17, -24 H -25), and other MMPs (MMP-19, -20, -28, -21, -22, -23)(13).
Numerous authors have established a positive correlation between MMP expression patterns and the invasive and metastatic potential of a variety of tumor types, including rectal and gastric cancer, lung carcinoma, breast, ovarian, prostate, thyroid and brain tumors.
Tissue inhibitors of MMPS (TIMPs) expression is upregulated in response to tumor progression, resulting in MMP suppression and ECM integrity preservation.
Due to TIMPs’ dual role in activating pro-MMPs and MT1-MMP, it is possible that the activator/inhibitor ratio defines tumor growth and metastasis.
MMP-1
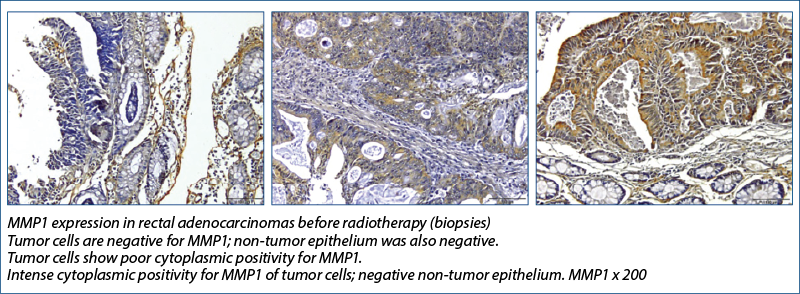
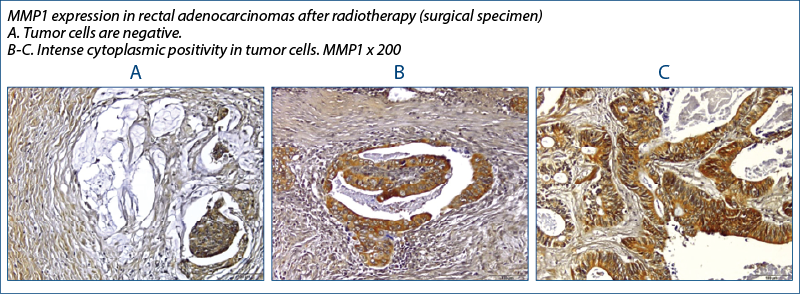
Also known as interstitial collagenase or fibroblastic collagenase, the MMP1 molecule has a pre-domain, a pro-domain, a catalytic domain, a binding region and a hemopexin-like domain, with a role in the degradation of collagen types I, II and III. MMP-1 in the tumor and mucosa was associated with an increased risk of metastasis.
Matrix metalloproteinase-1 is involved in the regulation of the extracellular matrix, and its genetic role in colorectal cancer (CRC) is unknown. The purpose of a study made by Wu et al.(14) was to determine the role of matrix metalloproteinase-1 genotypes in the risk of colorectal cancer in Taiwan. The research concluded that MMP-1 rs1799705 genotypes contribute to susceptibility to CRC risk.
Angenete et al.(15) investigated the effect of radiotherapy on the rectal mucosa and extracellular matrix (ECM) of rectal tumors by examining the enzymes and growth factors involved in ECM remodeling and found that, prior to irradiation, tumor tissues contained significantly more MMP-1, -2, -9, total TGF-beta1, uPA, PAI-1, and calprotectin than mucosa, whereas TIMP-1 and the active TGF-beta-1 fraction remained unchanged.
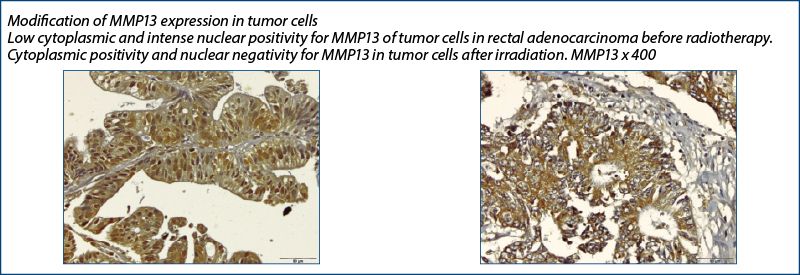
MMP-13
A study performed by Roeb et al.(16) had as a purpose to perform a semiquantitative reverse transcriptase-polymerase chain reaction to compare MMP-7 gene expression to that of MMP-1, MMP-3 and MMP-13 in patients with resectable rectal and colon cancer (RT-PCR) and found that the majority of cases of colorectal carcinogenesis were associated with increased expression of MMP-7, -13, -3, whereas MMP-1 expression was highly variable and often not overexpressed. The inhibition of MMP-7, as well as of MMP-13 and MMP-3, may be beneficial as a preventive or therapeutic adjunct in colorectal cancer.
TIMP1
It is a glycoprotein, with the roles of: 1) natural inhibitor of MMP proteins (matrix metalloproteinases = group of peptidases involved in the metabolism and degradation of extracellular matrix), 2) promoter of cell proliferation for many cell types, and 3) it could have an anti-apoptotic role.
Among the research looking into an analysis between the level of TIMP 1 and the tumor response and survival to preoperative radiochemotherapy in rectal cancer patients, we mention the one performed by Oblak et al.(17) Another study conducted by Yoon et al.(18) analyzed the role of biomarkers such as serum tissue inhibitor of metalloproteinases-1 (TIMP-1) in predicting the pathologic response to rectal cancer neoadjuvant chemoradiation (NACRT) and the findings indicated that serum TIMP-1 levels following NACRT could be used to predict the pathologic response to NACRT in rectal cancer, even in patients who achieved clinical response.
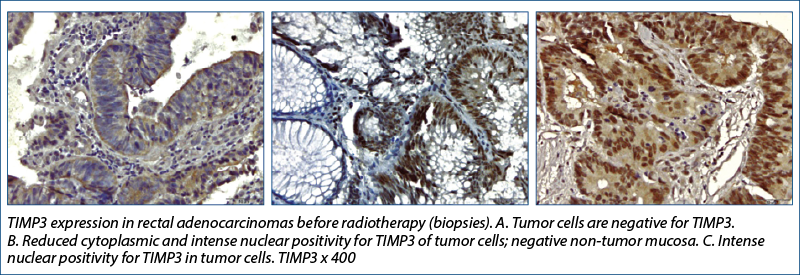
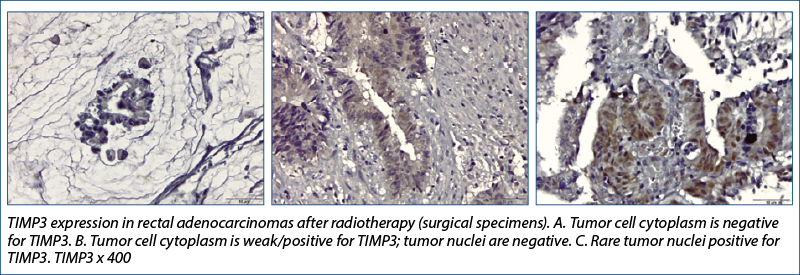
TIMP-3
Research conducted by Molinari et al.(19) showed that TIMP3 methylation status differed significantly between the four tumor regression grade classes (ANOVA, p=0.05) when the association between methylation and the effects of therapy on tumor samples were analyzed (evaluating the tumor response to radiochemotherapy in rectal cancer).
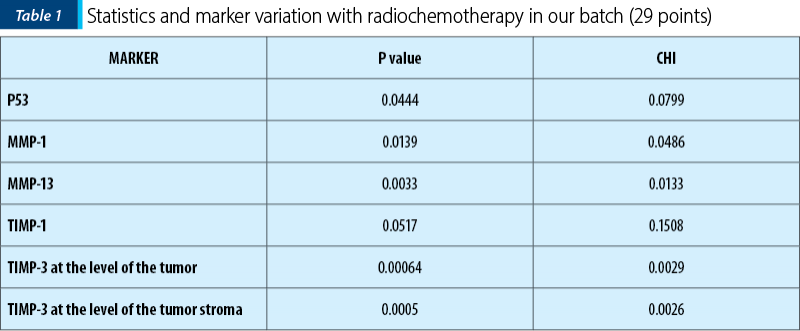
In conclusion, from the analysis of the group of 29 patients in whom other immunohistochemical markers were studied and their modifications with radiotherapy, the following markers showed significant variation.
P53 varied its expression, involving the modification of the cellular repair behavior in case of cellular exposure to stress, in this case the “threat” of cellular injury being brought by the radiotherapy itself.
MMP1 has increased expression with radiotherapy, also of statistically relevant value and since MMP1 is involved in extracellular matrix degradation and metastasis, then the immediate clinical involvement would be to be able to detect if radiotherapy does not increase the metastatic potential of cells, through this mechanism
MMP13 became negative in most tumor cell nuclei of the tumor stroma after radiotherapy – after being initially positive (pre-irradiation) –, a phenomenon which could demonstrate an opposite effect to the previous one, namely the involvement of radiotherapy in decreasing the oncogenic potential.
TIMP-1 can promote cell proliferation and, in some cases, may even acquire antiapoptotic function. In our group, TIMP 1 was slightly positive with tumor radiotherapy, a result that is consistent with the results related to MMP-1
TIMP-3 lost its positivity (most values changed from positive to 0) and the change was statistically significant, both at the level of the tumor and of the tumor stroma. The expression of this gene generally correlated with the mitogenic response and its decrease with radiation therapy would lead to the assumption that radiation therapy would inhibit tumor cell proliferation through this mechanism.
The study is currently underway, with all the aforementioned elements being preliminary results.
Considering these aspects, the closing argument of the present article would be that radiotherapy can have both positive (p53, MMP13, TIMP3) and negative (MMP1, TIMP1) implications on the behavior of tumors, normal epithelium and tumor and normal stroma. Being able to correlate these changes with a clear prognosis and clinical outcome would imply the study of the biomarkers in the same group (and/or at the same time) in which patient monitoring is performed. The targets envisaged to enhance the value of the research would be to continue it with a prospective comparative study in which to detect patient survival, disease-free interval and perioperative morbidity. All in the context of measuring the response of immunohistochemistry markers to radiochemotherapy.
Acknowledgement: Part of the initial research in the article was conducted by Dr. Ionescu Sânziana during the PhD thesis “Răspunsul tumorilor de rect la radioterapie” (“Tissue response to radiotherapy in rectal cancer”), under the supervision of Prof. Dr. Eugen Brătucu from the “Prof. Dr. Alexandru Trestioreanu” Institute of Oncology Bucharest and the “Carol Davila” University of Medicine and Pharmacy, Bucharest. The authors would like to thank the pathology department of the Colentina Clinical Hospital, under Prof. Dr. Sabina Zurac and Prof. Dr. Florica Stăniceanu’s supervision, for their invaluable and much-appreciated contribution to the realization of this study.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Kim NK, Hur H. New Perspectives on Predictive Biomarkers of Tumor Response and Their Clinical Application in Preoperative Chemoradiation Therapy for Rectal Cancer. Yonsei Med J. 2015 Nov;56(6):1461-77.
-
Liu SL, O’Brien P, Zhao Y, Hopman WM, Lamond N, Ramjeesingh R. Adjuvant treatment in older patients with rectal cancer: a population-based review. Curr Oncol. 2018 Dec;25(6):e499-e506.
-
Alecu M, Simion L, Straja N, Brătucu E. Multiple polyps and colorectal cancer. Chirurgia (Bucur). 2014 May-Jun;109(3):342-6. Available at: https://pubmed.ncbi.nlm.nih.gov/24956339/
-
Khakoo S, Carter PD, Brown G, Valeri N, Picchia S, Bali MA, Shaikh R, Jones T, Begum R, Rana I, Wotherspoon A, Terlizzo M, von Loga K, Kalaitzaki E, Saffery C, Watkins D, Tait D, Chau I, Starling N, Hubank M, Cunningham D. MRI Tumor Regression Grade and Circulating Tumor DNA as Complementary Tools to Assess Response and Guide Therapy Adaptation in Rectal Cancer. Clin Cancer Res. 2020 Jan 1;26(1):183-192.
-
Park YJ, Oh BR, Lim SW, Huh JW, Joo JK, Kim YJ, Kim HR. Clinical significance of tumor regression grade in rectal cancer with preoperative chemoradiotherapy. J Korean Soc Coloproctol. 2010 Aug;26(4):279-86.
-
Kim NK, Hur H. New Perspectives on Predictive Biomarkers of Tumor Response and Their Clinical Application in Preoperative Chemoradiation Therapy for Rectal Cancer. Yonsei Med J. 2015 Nov;56(6):1461-77.
-
Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010 Dec;222(4):350-66.
-
Zhao Y, Li X, Kong X. MTHFR C677T Polymorphism is Associated with Tumor Response to Preoperative Chemoradiotherapy: A Result Based on Previous Reports. Med Sci Monit. 2015 Oct 12;21:3068-76.
-
Characterization of TP53 polymorphisms in Romanian colorectal cancer patients (PDF). Available at: https://www.researchgate.net/publication/330183727_Characterization_of_TP53_polymorphisms_in_Romanian_colorectal_cancer_patients (accessed Dec. 07, 2021).
-
Yang J, Lin Y, Huang Y, Jin J, Zou S, Zhang X, Li H, Feng T, Chen J, Zuo Z, Zheng J, Li Y, Gao G, Wu C, Tan W, Lin D. Genome landscapes of rectal cancer before and after preoperative chemoradiotherapy. Theranostics. 2019 Sep 21;9(23):6856-6866.
-
Huang A, Xiao Y, Peng C, Liu T, Lin Z, Yang Q, Zhang T, Liu J, Ma H. 53BP1 expression and immunoscore are associated with the efficacy of neoadjuvant chemoradiotherapy for rectal cancer. Strahlenther Onkol. 2020 May;196(5):465-473.
-
El Otmani I, El Agy F, El Baradai S, Bouguenouch L, Lahmidani N, El Abkari M, Benajah DA, Toughrai I, El Bouhaddouti H, Mouaqit O, Ibn Majdoub Hassani K, Mazaz K, Benjelloun EB, Ousadden A, El Rhazi K, Bouhafa T, Benbrahim Z, Ouldim K, Ibrahimi SA, Ait Taleb K, Chbani L. Analysis of Molecular Pretreated Tumor Profiles as Predictive Biomarkers of Therapeutic Response and Survival Outcomes after Neoadjuvant Therapy for Rectal Cancer in Moroccan Population. Dis Markers. 2020 Jan 11;2020:8459303.
-
Velinov N, Poptodorov G, Gabrovski N, Gabrovski S. [The role of matrixmetalloproteinases in the tumor growth and metastasis]. Khirurgiia (Sofiia). 2010;(1):44-9. Bulgarian.
-
Wu MH, Yueh TC, Chang WS, Tsai CW, Fu CK, Yang MD, Yu CC, Bau DT. Contribution of Matrix Metalloproteinase-1 Genotypes to Colorectal Cancer in Taiwan. Cancer Genomics Proteomics. 2021 May-Jun;18(3):245-251.
-
Angenete E, Oresland T, Falk P, Breimer M, Hultborn R, Ivarsson ML. Preoperative radiotherapy and extracellular matrix remodeling in rectal mucosa and tumour matrix metalloproteinases and plasminogen components. Acta Oncol. 2009;48(8):1144-51.
-
Roeb E, Arndt M, Jansen B, Schumpelick V, Matern S. Simultaneous determination of matrix metalloproteinase (MMP)-7, MMP-1, -3, and -13 gene expression by multiplex PCR in colorectal carcinomas. Int J Colorectal Dis. 2004 Nov;19(6):518-24.
-
Oblak I, Velenik V, Anderluh F, Mozina B, Ocvirk J. The correlation between the levels of tissue inhibitor of metalloproteinases 1 in plasma and tumour response and survival after preoperative radiochemotherapy in patients with rectal cancer. Radiol Oncol. 2013 May 21;47(2):138-44.
-
Yoon HI, Koom WS, Kim YB, Min BS, Lee KY, Kim NK, Shin SJ, Ahn JB, Keum KC. Predicting the pathologic response of locally advanced rectal cancer to neoadjuvant concurrent chemoradiation using enzyme-linked immunosorbent assays (ELISAs) for biomarkers. J Cancer Res Clin Oncol. 2014 Mar;140(3):399-409.
-
Molinari C, Casadio V, Foca F, Zingaretti C, Giannini M, Avanzolini A, Lucci E, Saragoni L, Passardi A, Amadori D, Calistri D, Zoli W. Gene methylation in rectal cancer: predictive marker of response to chemoradiotherapy? J Cell Physiol. 2013 Dec;228(12):2343-9.