Bone metastases (BM) are experienced nearly by one of two of all cancer patients either at presentation or during the disease course. As a fundamental rule, because treatment of BM usually aims to palliate, the cardinal objectives of treatment should incorporate the attainment of a rapid and durable pain relief with preserved mobility and functionality without causing significant toxicity. With its favorable toxicity profile and an overall response rate of greater than 80%, radiotherapy (RT) is a well-recognized as a palliative measure for treatment of BM. The present article will exclusively focus on the role of RT in the management of BM with a specific emphasis on the ongoing debate of fractionation and total dose schemes.
Rolul radioterapiei în managementul metastazelor osoase
Management of bone metastases with radiation therapy
First published: 24 martie 2017
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
Metastazele osoase (MO) sunt întâlnite la unu din doi pacienți oncologici, de la prezentare sau pe parcursul evoluției bolii. Ca o regulă fundamentală, pentru că tratamentul MO are de obicei scop paleativ, obiectivele principale ale tratamentului ar trebui să includă obținerea unui efect antalgic rapid și de durată, cu păstrarea mobilității și a funcționalității, și fără a cauza toxicități majore. Cu profilul de toxicitate favorabil și cu rate de răspuns global de peste 80%, radioterapia (RT) este recunoscută ca un tratament paleativ eficient în MO. Acest articol se va concentra exclusiv pe rolul RT în managementul MO, cu o atenție specială pe dezbaterea continuă legată de fracționare și doze totale ale diferitelor protocoale de tratament.
Introduction
Approximately 50% of all patients with cancer diagnosis will experience BMs either at presentation or somewhere during their disease course. One-third of patients with lung cancer and between one-third to two-thirds of those with breast or prostate cancer will develop BM, which altogether accounts for 80% of all BM(1). Among other cancers, BMs are often present also in thyroid and renal cancer and multiple myeloma. Prevalence of BM has a tendency to further increment in parallel with the innovative advances in cancer diagnosis, imaging modalities, and implementation of more effective anticancer treatments, which prompts prolonged survival and resultant emergence of further metachronous BMs.The primary aim of this review article is to exclusively focus on the role of RT in the management of BM with a specific emphasis on the ongoing debate of fractionation and total dose schemes.
Pathophysiology of BM
According to Paget’s “seed-and-soil” hypothesis proposed for BM in 1889(2), other than being an extremely fertile soil for the growth of tumor cells, bone is the largest storage site of growth factors in the body, which are activated when BM occurs. Further, stromal cells of bone marrow express chemotactic factors that attract tumor cells to bone and then provide a supportive microenvironment for tumor cells to grow.While the biology of BM is a multistep complex process involving a multitude of factors and growth pathways, yet it can be summarized briefly as follows. Cancer cells home to endosteal niches in the marrow when BM occurs, which is facilitated by a group of factors including SDF-1, AXII, and RANKL proteins expressed by both osteoblasts and stromal cells(3-5). Osteoclasts play key roles in BM by inducing tumor mobilization through release of MMP-9, cathepsin-K, osteopontin, and stem cell factor into the neighboring bone marrow(6).
Traditionally, although both processes occur in most types except for the BM of exclusively osteolytic multiple myeloma, BM are classified as osteolytic and osteoblastic variants which represent for bone destruction and bone formation as the dominant feature. Osteoclasts mediate increased bone resorption that occurs in both osteolytic and osteoblastic BM. The osteolytic bone destruction process is activated and progressed by the release of growth factors from bone, including calcium TGF-α, IGF-1 and -2, FGF, PDGF, and bone morphogenetic proteins(7). Osteoblastic factors are either produced or induced by tumors cells, enrich the local tumor cell microenvironment and stimulate osteoblast proliferation, differentiation, and the secretion of additional growth factors. Marrow stromal cells and osteoblasts additionally promote tumor cell growth by means of production of growth factors and cytokines. Osteoclast differentiation factor RANKL is the main pathway that most of the tumor cell originated osteolytic factors act by and increase osteoclastic proliferation, maturation, and survival, which ends up with bone resorption. Besides the close interactions between the BM cells and bone marrow matrix which enhance the further tumor growth, the bone marrow microenvironment also promotes chemo- and radiation resistance by keeping tumor stem cells in a dormant state. As has been argued more than 50 years before, certain bone morrow derived growth factors may further fertilize a specific region of bone for BM growth and may explain the differential distribution of BM of various tumor types in the skeleton(8).
Symptomatology of BM
Approximately 50-70% of BM patients present with various symptoms including bone pain, pathological fractures, hypercalcaemia, nerve-root damage or compression, and spinal cord (SCC)(1). Since BM as a rule include multiple bone sites with only less than 10% being solitary lesions, patients may experience simultaneously presenting multiple symptoms in connection with the included sites.Bone pain is the most common complication and usually the first symptom of BM that is experienced approximately by 75% of all BM patients and induced by a complex mechanism that incorporates a combination of mechanical and biochemical factors(9). Pain is experienced most commonly at axial skeletal bones as 80% of the BM involves these sites (mainly vertebral column and/or pelvis). In addition to neuropathic pain, stress fracture in the form of micro- or macro-fractures may also contribute to bone pain. Although the exact analgesic mechanism of RT has not been understood in details until now, yet 75% to 85% of all BM patients demonstrate an objective pain response to RT characterized with reduction/discontinuation in analgesic usage: 25% within 2 days, 50% within 4 weeks, and 25% later than 4 weeks from the first day of RT(10).
Pathological fractures, a significant cause of morbidity, occur in 8% to 30% of BM patients and usually involve the weight bearing bones such as the femur, vertebrae, and pelvic bones. Therefore, the assessment of a patient with BM should include thorough evaluations for presence of pathologic fractures. Surgical stabilization or vertebroplasty should be considered for suitable patients before fractionated RT of 20 to 30 Gy, which will be beneficial in enhancing recovery process and reducing the persistent pain caused by residual tumor cells(11,12). RT may also be useful in prevention of future fractures of asymptomatic but involved bones by reducing the tumor burden and promoting normal bone regrowth. Fractionated RT is preferred for palliative treatment of such bones because of the concerns related with RT-induced pathologic fractures with higher dose single fraction regimens, however, the results of benchmark Radiation Therapy Oncology Group (RTOG) 97-14 trial showed that there was no difference between the single fraction 8 Gy and the 30 Gy given in 10 fractions regimens regarding long-term pathologic fracture risks(13).
Despite the fact that other symptoms such as hypercalcemia (10%), nerve root damage/compression or SCC (5%) may be less frequently encountered, yet their earlier diagnosis and appropriate treatment is crucial in order to enhance or at least to stabilize the health related quality of life measures.
Diagnosis of BM
A through physical examination should be the first step of BM evaluations as a localized pain revealed on palpation may be beneficial in guidance of further diagnostic studies and the order of treatment in patients with multiple BMs. Feasibility of various biochemical bone turnover markers have been investigated in diagnosis of BM. However, notwithstanding of the way that they may add to standard imaging tools these markers were observed to be less sensitive than the direct radiological assessment of the skeleton utilizing plain radiographs, bone scintigraphy, computed tomography (CT), magnetic resonance imaging (MR), Single photon emission computer tomography (SPECT) or positron emission tomography-CT (PET-CT). Along these lines, imaging based assessments of bone lesions has turned into the mainstay of diagnostic BM evaluations(14).Plain radiography is not generally suggested as a screening strategy for introductory BM assessments due to its poor sensitivity, and thusly, is most usually used to assess symptomatic sites and to affirm findings on other imaging studies. Albeit bone scintigraphy is more sensitive than plain radiographs, it suffers from the lack of acceptable specificity as the tracer accumulation may occur in any skeletal lesion with an elevated rate of bone turnover, such as accompanying traumas, infection or arthropathy(15).
Bone CT has been exhibited to be more sensitive than bone scintigraphy, especially in certain conditions. For instance, considering the fact that degenerative joint diseases are well-known sources of increased tracer uptake in bone scintigraphy, CT is superior over bone scintigraphy in differentiating BM from degenerative joint disease even though both may coexist(16). CT is valuable also in demonstration of small intramedullary lesions and soft-tissue involvement.
Single photon emission computer tomography (SPECT) has improved the true detectability of BM by its higher sensitivity and specificity. The tomographic presentations of SPECT images have fortified the clinicians’ capacity to detect abnormal tracer uptake in involved bones, particularly in the dense parts of the body such as the spine and pelvis. This feature has also improved our ability regarding the discrimination of metastatic foci from other anomalies prompting increased localized tracer uptake(17).
MRI has a high sensitivity in detection of BM involvement of the bone marrow. Albeit both T1- and T2-weighted images are required, MRI is especially appropriate to detect early spinal metastases within the medulla, and many authors agree that it is more sensitive than the bone scintigraphy for this purpose(18). Moreover, MRI is additionally helpful in discriminating malignant vertebral collapses from the benign ones. Disadvantages of MRI include its high cost, exclusion of patients with metal implants, claustrophobia, and lower capacity in the bone cortex compared to a CT scan(19).
PET-CT, with 18F-fluoride or 2-fluoro-deoxy-D-glucose tracers, is utilized for both the initial locoregional staging of malignancy of interest and in evaluation of presence of distant metastases including the BM. Compared to bone scintigraphy PET-CT has a similar sensitivity (74-95%), but a significantly higher specificity (90% to 97%)(20-24). However, PET-CT also suffers from some certain limitations as its BM detecting capacity relies on certain factors, such as, lesion size, tumor grade, tumor’s metabolic activity, the surrounding background activity, and the serum glucose levels(21,25).
In summary, albeit each diagnostic imaging tool brings its pros and cons together, considering their relatively higher sensitivities and specificities, MRI and PET-CT appear to be the most useful current imaging strategies for initial diagnostic assessment of BMs.
RT for BM
Treatment of BM patients with RT should be arranged individualized and well balanced, rather than being straightforward, by considering multiple patient and disease related variables including the needs of patient and overall prognosis. For instance, it should be recognized that patients with longer survival expectations may require a more aggressive treatment to attain durable pain relief, yet such a treatment maneuver may also be associated with long term treatment-related complications. The results of the benchmark RTOG-74-02 study exhibited superior median survival times in patients with solitary than multiple BM (36 vs. 24-week; p=0.005), and of those with breast and prostate primaries than lung cancer (30 to 73 vs. 12 to 14 weeks; p<0.0001) for both solitary and multiple BM groups(10), which lands strong support for the notion suggesting consideration of multiple covariates in order to accomplish an appropriate treatment plan for such patients.Search for the ideal RT fractionation scheme for BM has been the issue of greatest interest which has been investigated by various randomized control trials’ (RCTs) and meta-analyses(26-35) (Table 1). Between 1974 and 1980 the RTOG promoted a landmark randomized trial RTOG-74-02 to compare various dose-fractionation schedules for patients presenting with either solitary (n=266) or multiple BM (n=750)(10). The solitary BM group received 40.5 Gy /15 fx or 20 Gy/5 fx, while multiple BM group was randomized to one of 30 Gy/10 fx, 15 Gy/5 fx, 20 Gy/5 fx, or 25 Gy/5 fx arms. Results of this RCT demonstrated 95% objective efficacy with 54% being complete response in terms of pain relief. Overall median duration of complete pain relief was 12 weeks. Based on the absence of efficacy difference among the study arms, the authors concluded that all RT schedules were equally effective in palliation of pain(10). In 1985, Blitzer(36) reanalyzed the RTOG data with the end goals of investigating the influence of the number of fractions (single vs. multiple), dose per fraction, and number of metastases (solitary versus multiple) on the likelihood of accomplishing complete pain relief and requirement for retreatment. The results of this reanalysis differed from the previously published original report in that number of fractions was statistically significantly related to complete combined relief (that is, absence of pain and cessation of the use of narcotics). Also, the time dose factor isoeffect conversion did not accurately predict tumor response. Based on this report, Blitzer concluded that the protracted dose-fractionation schedules were more effective than short course schedules. However, although there exist some evidence supporting for longer duration of pain relief with protracted regimens, excluding the higher need for retreatment with single fraction RT, available literature for the most part suggest no notable difference between single fraction and protracted RT schedules in terms of palliative efficacy(36).
The issue of optimal RT fractionation has also been addressed in four meta-analyses(26-29). In the first meta-analysis, Wu et al.(26) identified two trials comparing single versus single, eight trials comparing single versus multiple, and six trials comparing multiple versus multiple fractions. The authors reported that there was no significant difference in complete and overall pain relief between single and multifraction RT arms. Additionally, no dose-response relationship was detected in the multifraction versus multifraction trials. The second meta-analysis, by Sze et al.(27), included 3435 BM patients from eleven trials.
Outcomes of this study suggested that single fraction RT was as effective as multifraction RT in relieving BM-associated pain. However, the re-treatment rate and pathological fracture rates were higher after single fraction RT. The third meta-analysis, by Chow et al.(28), included 16 trials and reported that there were no significant differences between the single fraction and protracted RT regimens in terms of overall and complete response rates efficacy and RT-induced toxicities. However, again the likelihood of re-treatment was higher (2.5-fold; p<0.00001) in single fraction RT arm patients. The fourth meta-analysis, by Chow et al.(29), was an update of the previous report and incorporated 25 RCTs. Overall and complete response rates were again similar in both intention-to-treat and assessable patients.
Single and multiple fraction regimens provided equal pain relief; however, significantly higher retreatment rates (2.6-fold; p<0.00001) occurred in those receiving single fractions.
Based on the outcomes of aforementioned RCTs and meta-analyses, the evidence-based clinical practice guideline of the American Society for Radiation Oncology (ASTRO) recommends a single fraction as being more convenient for patients and their caregivers for uncomplicated BM(37), defined as:
a. Lesions without spinal cord compression, cauda equina compression, radicular bone pain, or extensive involvement (>3 cm) of the femoral cortex.
b. Lesions not requiring surgical stabilization.
c. Spinal lesions that have not been previously irradiated.
d. Lesions for which retreatment would not be excessively problematic
Additionally, American Board of Internal Medicine Foundation has specifically evaluated cancer treatment methods and tests that may be over-, or unnecessarily or potentially harmfully used. The analyses performed by the work group representing the ASTRO Clinical Affairs and Quality, Health Policy, and Government Relations Councils reported the ASTRO’s 5 recommendations for the Choosing Wisely campaign. The subtopic about the treatment recommendations for BM was concluded as: “Don’t routinely use extended fractionation schemes (>10 fractions) for palliation of BM”(38) based on the facts that:
a. Studies suggest equivalent pain relief following 30 Gy in 10 fractions, 20 Gy in 5 fractions, or a single 8 Gy fraction.
b. A single treatment is more convenient but may be associated with a slightly higher rate of retreatment to the same site.
c. Strong consideration should be given to a single 8 Gy fraction for patients with a limited prognosis or with transportation difficulties(37,39,40).
In summary, accessible literature in the forms of RCTs, meta-analyses, and cooperative groups’ recommendations suggest equal palliative efficacy with single fraction RT and multiple fraction RT regimens. Despite the reluctance against single fraction RT which is not evidence based, with its equal efficacy shorter course, RT may prove beneficial in treatment of BM patients regarding the unnecessary hospital visits and related psychological stress, and useless expenditure of national founds.
Complications of RT
Compared to conventional RT era, fortunately the radiation induced acute and late complications have gradually decreased based on the usage of more conformal RT techniques, such as three-dimensional conformal RT and intensity-modulated RT (Figure 1). The acute side effects of RT are generally mild, self-limiting, and simple to treat, which are predictable based on the region planned to be irradiated. The main systemic side effect is fatigue, while local side effects include radiation-induced dermatitis, gastrointestinal complaints such as mucositis, nausea, vomiting, esophagitis, and diarrhea depending on the localization of the target BM. Compared to multi-fraction regimens some certain acute side effects have been proposed to exclusively associate with single fraction high dose RT, such as a transient increase in bone pain, so called “pain flare”(13,33). However, in particular studies by Loblaw et al.(41) and Hird et al.(42), the “pain flare”, which was accounted in 20-40% of irradiated patients during the first few fractions of RT, did not exhibit any connection with the fractionation scheme, and was concluded to be consequence of rapid tumor cell kill induced by RT. Non-steroidal or steroidal anti-inflammatory medications can effectively treat or at least minimize this specific complication. Another important complication of BM irradiation is pathologic bone fractures which has an incidence rate of 4-5% according to RTOG 97-14 study(13,43). In the randomized controlled Dutch Bone Metastasis Study of palliative RT, high risk factors for predicting pathologic femoral fractures were determined as the axial cortical involvement >30 mm (p= 0.01), and circumferential cortical involvement >50% (p= 0.03) with no notable association with RT fractionation(44).Late complications of RT are rare. This may at least partly be associated with short life spans of BM patients after palliative RT which prevents manifestation of late complications. On the other hand, due to advances in cancer therapies, some patients with BM may live longer and in this manner may conceivably be at risk of radiation-induced late complications. Radiation myelopathy is one such serious but fortunately late complication with classical RT doses of 8 to 40 Gy administered in 1 to 20 fractions. Likewise, serious esophageal, brachial and lumbar plexial complications and vertebral compression fractures are extremely rare and are typically experienced with SRS as opposed to the conventional or three-dimensional conformal RT practices in the above specified dose-fractionation range.
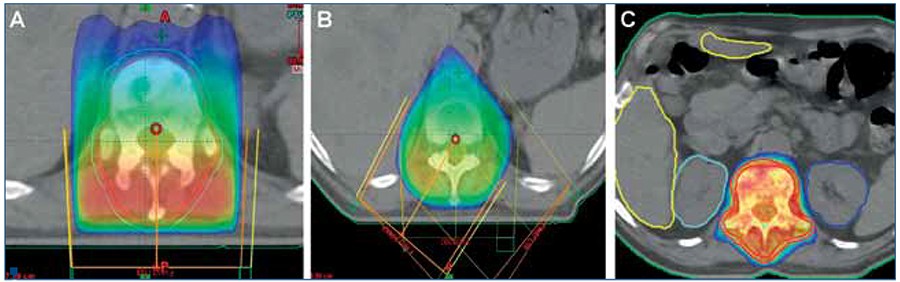
evident painful metastases localized to T12 and L1 vertebrae:
(A) Conventional single-field (RT); (B) Three-field three-dimensional RT; and (C) Intensity-modulated RT
Conclusion
Approximately half of all cancer patients will experience BM either at presentation or somewhere during their disease course. As a rule of thumb, since the treatment of BM is usually palliative, the main objectives of treatment should involve the achievement of a rapid yet durable pain relief with preservation of mobility and functionality in expense of minimal toxicity. With its minimal toxicity profile and an overall response rate of >80%, RT is a well-established palliative treatment measure for palliation of BM. Although the treatment decisions should be individualized, based on the results of RCTs, meta-analyses, and cooperative group recommendations, the current widely accepted standard of RT is ≤30 Gy, given in ≤10 fractions, with no efficacy difference between the single dose 8 Gy and multifractionated regimens. nBibliografie
2. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989; 8(2):98-101.
3. Christopher MJ, Liu F, Hilton MJ et al. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009; 114(7):1283-1284.
4. Shiozawa Y, Havens AM, Jung Y et al. Annexic II/annexinII receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008; 105:370-380.
5. Jones DH, Nakashima T, Sanchez OH et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006; 440(7084):692-696.
6. Kollet O, Dar A, Shivtiel S et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657-664.
7. Hauschka PV, Mavrakos AE, Iafrati MD et al. Growth factors in bone matrix.Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986; 261(27):12665-12674.
8. Cumming JD: A study of blood flow through bone marrow by a method of venous effluent collection. J Physiol 1962; 162:13–20.
9. Falkmer U, Järhult J, Wersäll P, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol.2003; 42(5-6):620-633.
10. Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: Final results of the study by the Radiation Therapy Oncology Group. Cancer 1982; 50:893–899.
11. Koswig S, Budach V. Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study. Strahlenther Onkol 1999; 175:500-508.
12. Townsend PW, Smalley SR, Cozad SC, et al. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys 1995; 31:43-49.
13. Hartsell WF, Scott C, Bruner DW, et al. Phase III randomized trial of 8 Gy in 1 fraction vs. 30 Gy in 10 fractions for palliation of painful bone metastases: preliminary results of RTOG 97-14. Int J Radiat Oncol Biol Phys 2003; 57:124.
14. Ulmert D, Solnes L, Thorek DLj. Contemporary approaches for imaging skeletal metastasis. Bone Res. 2015 Jul 14; 3:15024.
15. Jacobson AF, Stomper PC, Jochelson MS, Ascoli DM, Henderson IC, Kaplan WD. Association between number and sites of new bone scan abnormalities and presence of skeletal metastases in patients with breast cancer. J Nucl Med 1990; 31:387-392.
16. Muindi J, Coombes RC, Golding S, et al: The role of computed tomography in the detection of bone metastases in breast cancer patients. Br J Radiol 1983; 56:233–236.
17. Ryan PJ, Fogelman I. The bone scan: where are we now? Sem Nucl Med 1995; 25:76-91.
18. Daffner RH, Lupetin AR, Dash N, et al: MRI in the detection of malignant infiltration of bone marrow. AJR Am J Roentgenol 1986; 146:353–358.
19. Lauenstein TC, Goehde SC, Herborn CU, et al: Whole-body MR imaging: Evaluation of patients for metastases. Radiology 2004; 233:139–148.
20. Duarte PS, Zhuang H, Castellucci P, et al: The receiver operating characteristic curve for the standard uptake value in a group of patients with bone marrow metastasis. Mol Imaging Biol 2002; 4:157–160.
21. Gayed I, Vu T, Johnson M, et al: Comparison of bone and 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in the evaluation of bony metastases in lung cancer. Mol Imaging Biol 2003; 5:26–31.
22. Ohta M, Tokuda Y, Suzuki Y, et al: Whole body PET for the evaluation of bony metastases in patients with breast cancer: Comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun 2001; 22:875–879.
23. Qu X, Huang X, Yan W, Wu L, Dai K.A meta-analysis of ¹⁸FDG-PET-CT, ¹⁸FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur J Radiol. 2012; 81(5):1007-1015.
24. Hamaoka T, Madewell JE, Podoloff DA, et al: Bone imaging in metastatic breast cancer. J Clin Oncol 2004; 22:2942–2953.
25. Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011; 31(1):3-13.
26. Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003; 55:594-605.
27. Sze WM, Shelley MD, Held I, et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy a systematic review of randomized trials. Clin Oncol (R Coll Radiol) 2003; 15:345-352.
28. Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007; 25:1423-1436.
29. Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys 2012; 82:1730-1737.
30. El-Shenshawy H, Kandeel A, El-Essawy S. The effect of a singlefraction compared tomultiple fractions radiotherapy on painfulbone metastases with evaluation of computed tomographybone density in osteolytic bone metastases. Bull Alex Fac Med2006; 42:439.
31. Hamouda WE, Roshdy W, Teema M. Single versus conventional fractionated radiotherapy in the palliation of painful bone metastases. Gulf J Oncol 2007; 1:35-41.
32. Safwat E, El-Nahas T, Metwally H, et al. Palliative fractionated radiotherapy for bone metastases clinical and biological assessment of single versus multiple fractions. J Egypt Natl Cancer Inst 2007; 19:21-27.
33. ForoArnalot P, Fontanals AV, Galceran JC, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 2008; 89:150-155.
34. Amouzegar-Hashemi F, Behrouzi H, Kazemian A, et al. Single versus multiple fractions of palliative radiotherapy for bone metastases: a randomized clinical trial in Iranian patients. Curr Oncol 2008; 15:151.
35. Sande TA, Ruenes R, Lund JA, et al. Long-term follow-up of cancer patients receiving radiotherapy for bone metastases: results from a randomized multicentre trial. Radiother Oncol 2009; 91:261-266.
36. Blitzer PH: Reanalysis of the RTOG study of the palliation of symptomatic osseous metastasis. Cancer 1985; 55:1468–1472.
37. Lutz S, Berk L, Chang, E, et al. [April 11, 2014] Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;15; 79(4):965-976.
38. Hahn C, Kavanagh B, Bhatnagar A, et al. Choosing wisely: the American Society for Radiation Oncology's top 5 list. Pract Radiat Oncol. 2014; 4(6):349-355.
39. Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: A randomized, controlled, non-inferiority trial. Lancet Oncol. 2013; 15:164-171.
40. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: A subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013; 119:888-896.
41. Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007; 15:451-455.
42. Hird A, Chow E, Zhang L, et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three Canadian cancer centers. Int J Radiat Oncol Biol Phys 2009; 75:193-197.
43. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005; 97:798-804.
44. Van der Linden YM, Dijkstra PD, Kroon HM, et al. Comparative analysis of risk factors for pathological fracture with femoral metastases.J Bone Joint Surg Br 2004; 86:566-573.
Articole din ediţiile anterioare
Clinical and therapeutic particularities in dermatofibrosarcoma protuberans – a case report
Dermatofibrosarcomul protuberans (DFSP) este o formă rară de cancer cutanat, caracterizată prin agresivitate locală, întâlnită cu predilecţie la po...
Tratamentul paliativ şi suportiv în cancerul bronhopulmonar avansat
Lucrarea caută să prezinte concis aspecte esenţiale în terapia paliativă şi suportivă a cancerului bronhopulmonar avansat. Se face distincţia între...
Evoluţia raportului neutrofile-limfocite (NLR) şi a raportului trombocite-limfocite în timpul polichimioterapiei în cancerele capului şi gâtului
Identificarea unor biomarkeri prognostici şi predictivi în patologia oncologică este un deziderat al cercetării translaţionale în cancer.
Diffuse large B-cell lymphoma and metachronous metastatic renal cancer – a case report
Dintre tumorile tractului urinar, cancerul renal este cel mai frecvent, iar în ceea ce priveşte subtipul histopatologic, cel cu celule clare ocupă ...