Advanced non-small cell lung cancer (NSCLC)
Personalized therapy
Patient-related factors
-
Performance status
-
Co-morbidity, organ functions
-
Age
-
Gender
-
Side effects of drugs
-
Convenience of administration
-
Patient preference
Tumor-related factors
-
Histological subtype
-
Tumor growth
-
Molecular characteristics
Costs, cost effectiveness, value-based judgements
Signature and mutational processes in human cancer
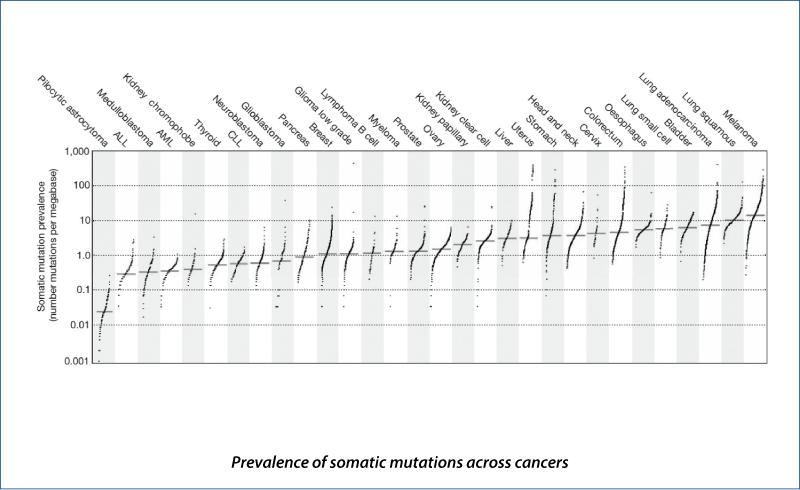
(Alexandrov LB et al. Nature, 2013, 500, 415)
Driver mutations
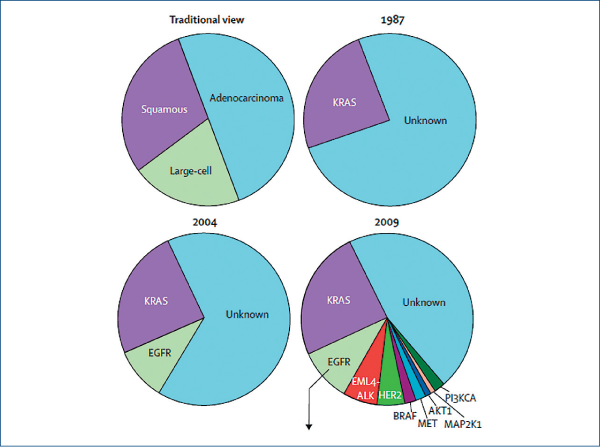
(Pao W and Girard N et al. Lancet Oncol, 2011, 12, 175)
Advanced NSCLC
Status September 2015
EGFR mutation-positive tumors
-
30-60% of Asian patients (adenocarcinomas)
-
10-15% of Caucasian patients (adenocarcinomas)
ALK-positive tumors
-
3-5% of patients (adenocarcinomas)
Tumors without targetable driver mutations
-
Majority of Caucasian patients with advanced NSCLC
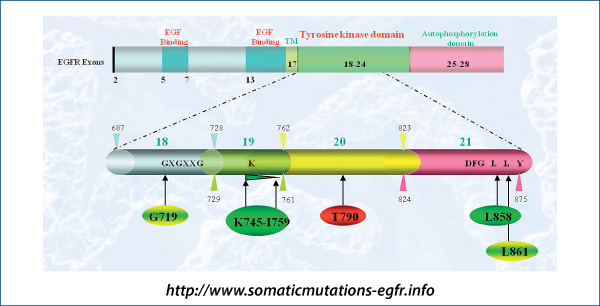
EGFR mutations and response to TKIs

First-line EGFR TKIs vs. chemotherapy in patients with EGFR mutation
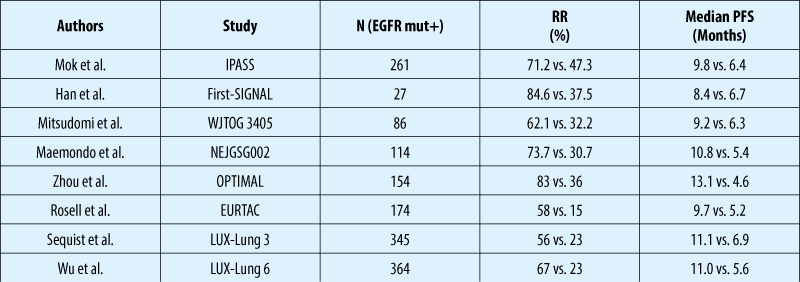
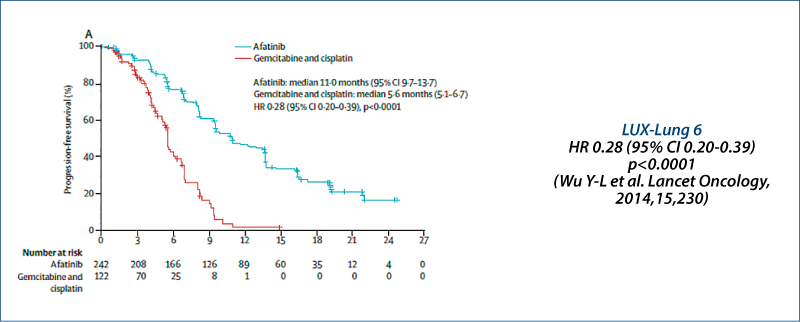
LUX-Lung 3 and LUX-Lung 6: PFS
Independent review
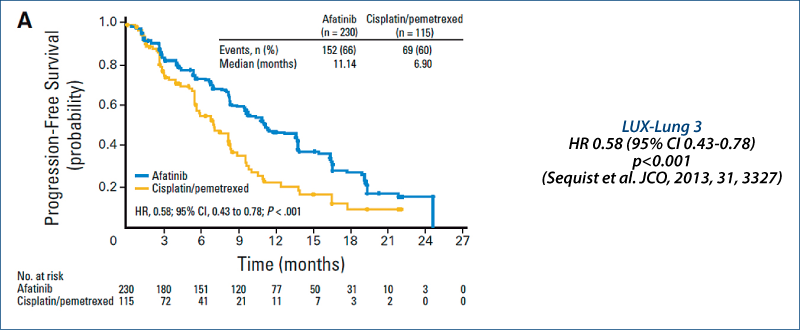
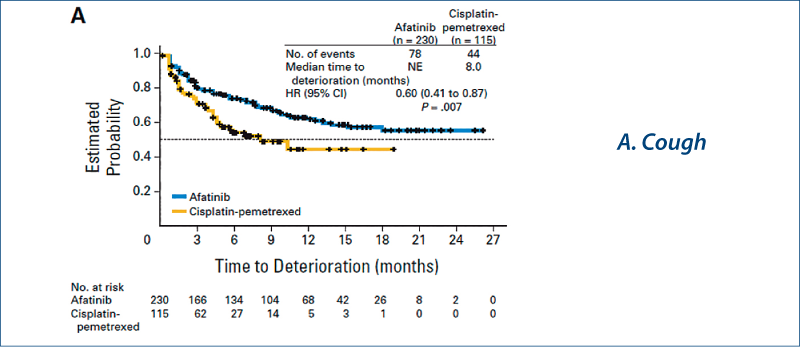
LUX-Lung 3: Quality of life
(Yang JC-H et al. JCO, 2013, 31, 3342)
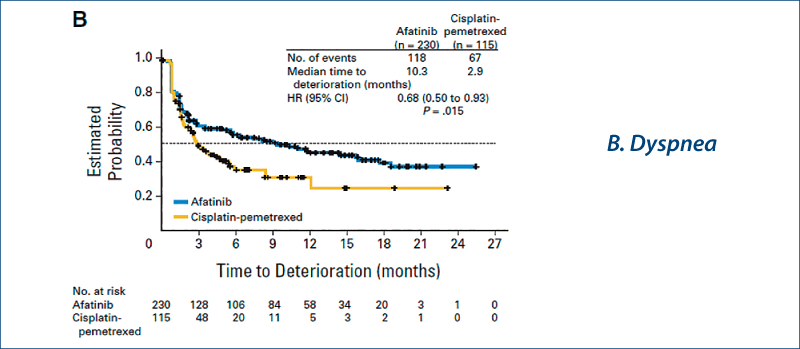
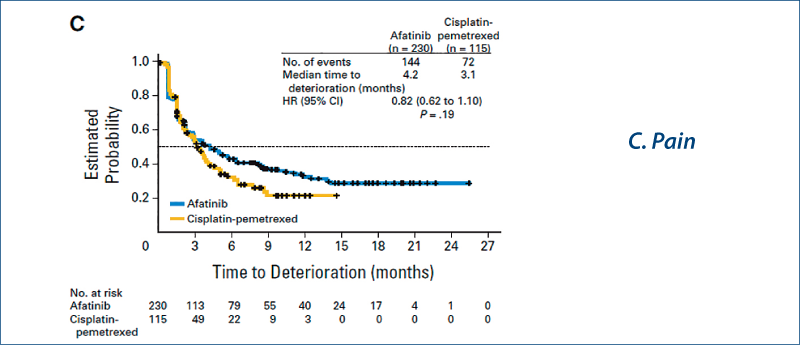
Afatinib vs chemotherapy for EGFR mutation-positive lung adenocarcinoma:
LUX-Lung 3 and LUX-Lung 6
(Yang JC-H et al. Lancet Oncol, 2015, 16, 141)
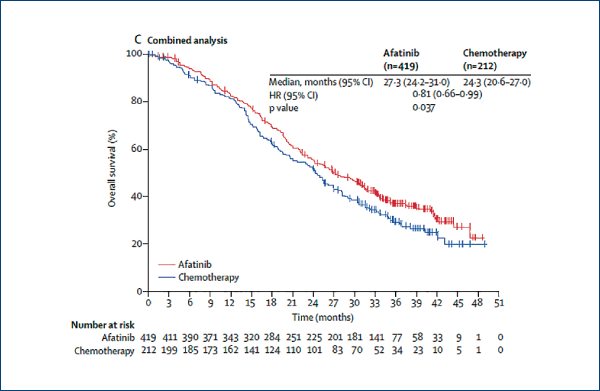
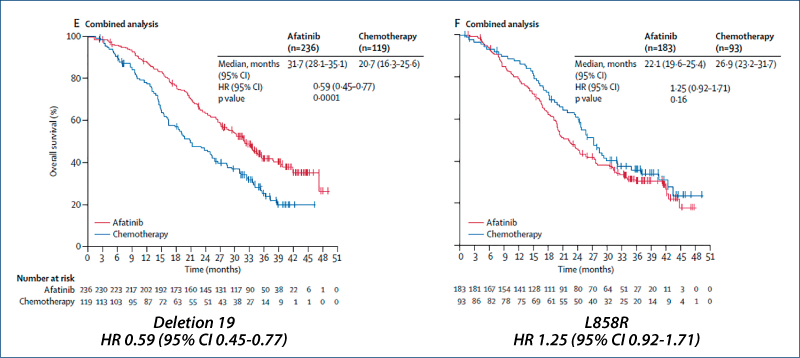
Afatinib vs. chemotherapy for EGFR mutation-positive lung adenocarcinoma:
LUX-Lung 3 and LUX-Lung 6
(Yang JC-H et al. Lancet Oncol, 2015, 16, 141)
Lung cancer
Molecular diagnosis in Europe
EGFR mutation testing has been established
-
European Workshop (Pirker R et al., JTO 2010, 5, 1706)
-
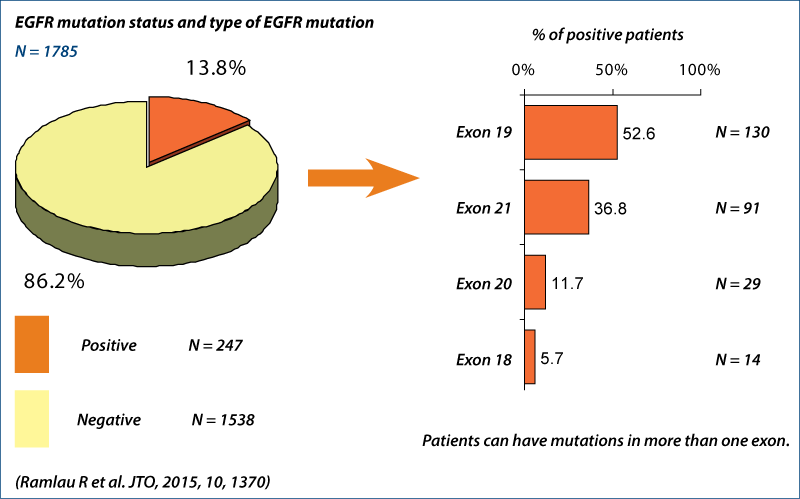
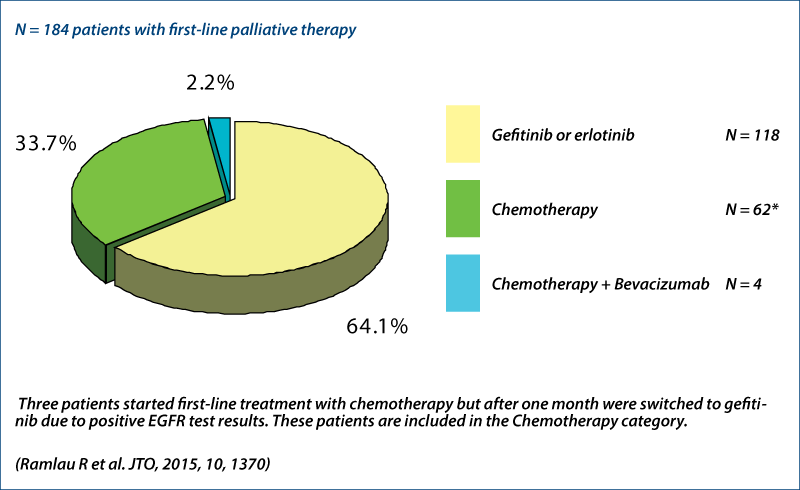
INSIGHT project (Ramlau R et al., JTO 2015, 10, 1370)
Routine KRAS testing in some countries, e.g. Hungary
-
FR TKIs are approved only for KRAS wild-type patientsEG
-
EGFR analysis only in KRAS wild-type patients
ALK analysis
-
IHC screening
-
FISH
French experience (Barlesi F et al., ASCO 2014)
EGFR mutation status
First-line therapy
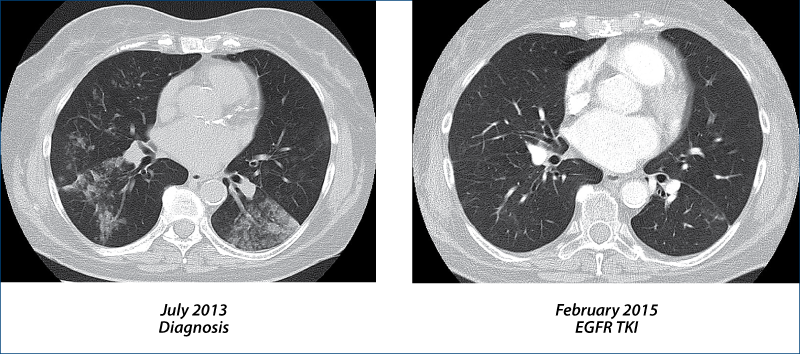
Adenocarcinoma with exon 19 deletion
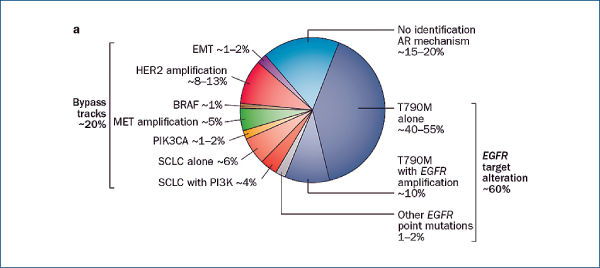
EGFR TKI-resistant NSCLC
(Camidge R et al. Nat Rev Clin Oncol, 2014, 11, 473)
Treatment at time of TKI resistance
Re-biopsy
Treatment
-
Third generation EGFR TKI
- AZD9291, rociletinib (CO-1686), HM61713
-
Switch to chemotherapy with potential re-challenge with TKIs after chemotherapy
-
Continue with TKI
-
Add local therapy to TKI
-
Add chemotherapy to TKI
-
Afatinib plus cetuximab
AZD9291 in EGFR inhibitor-resistant NSCLC
(Jänne PA et al. NEJM, 2015, 372, 1689)
Selective third generation TKI
EGFR mutation-positive patients with acquired resistance to EGFR TKIs (NCT01802632)
Dose escalation and dose expansion cohorts
20-240 mg AZD9291 once daily
Patients (n=253)
Female 62%
Asian 62%
Adenocarcinoma 96%
Prior TKIs median 2 (range 1-5)
Prior chemotherapy 80%
T790M 62%
AZD9291 in EGFR inhibitor-resistant NSCLC
(Jänne PA et al. NEJM, 2015, 372, 1689)
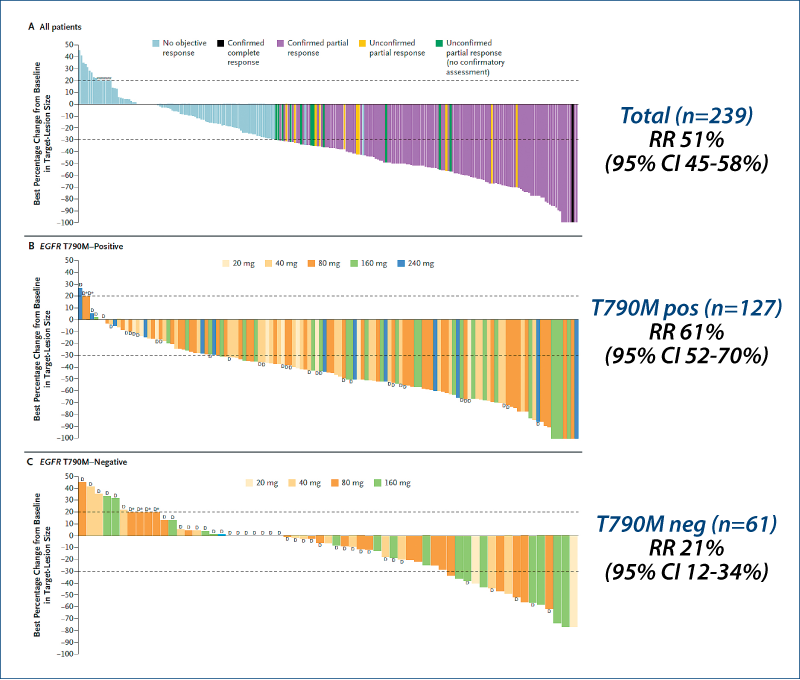
AZD9291 in EGFR inhibitor-resistant NSCLC
(Jänne PA et al. NEJM, 2015, 372, 1689)
No dose-limiting toxicity
AE any grade (grade 3-5)
Diarrhea 47% (2%)
Rash 40% (1%)
Dry skin 20% (0)
Puritus 19% (0)
Paronychia 17% (<1%)
Nausea 22% (<1%)
Decreased appetite 21% (1%)
Fatigue 17% (1%)
AE grade 3-5 32%
AE gr 3-5 drug-related 13%
SAE 22%
SAE drug-related 6%
AZD9291: Clinical studies in EGFR
mutation-positive NSCLC
AURA phase I/II study
AURA 2
-
Phase II trial
AURA 3
-
Phase III trial AZD9291 vs. platin-based chemotherapy in second-line therapy
FLAURA
-
Phase III trial AZD9291 vs. gefitinib or erlotinib in first-line therapy
ADAURA
-
Phase III trial AZD9291 vs. placebo as maintenance therapy
ASTRA
-
AZD9291 in early access program
Rociletinib in EGFR-mutated NSCLC
(Sequist LV et al. NEJM, 2015, 372, 1700)
Selective irreversible 3rd generation drug (activating and T790 mutations)
Phase I study in previously treated patients with EGFR mutation-positive disease (NCT01526928)
CO-1686 free base form up to 900 mg twice daily
CO-1686 hydrogen bromide form 500-1000 mg twice daily
Patients (n=130)
-
Female 77%
-
Asian 15%
-
Prior therapies median 4
-
Prior TKI median 2
-
T790M 57%
Rociletinib in EGFR-mutated NSCLC
(Sequist LV et al. NEJM, 2015, 372, 1700)
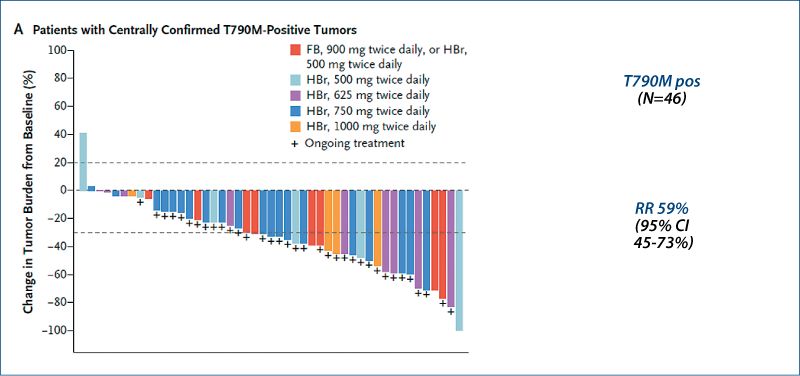
Rociletinib in EGFR-mutated NSCLC
(Sequist LV et al. NEJM, 2015, 372, 1700)
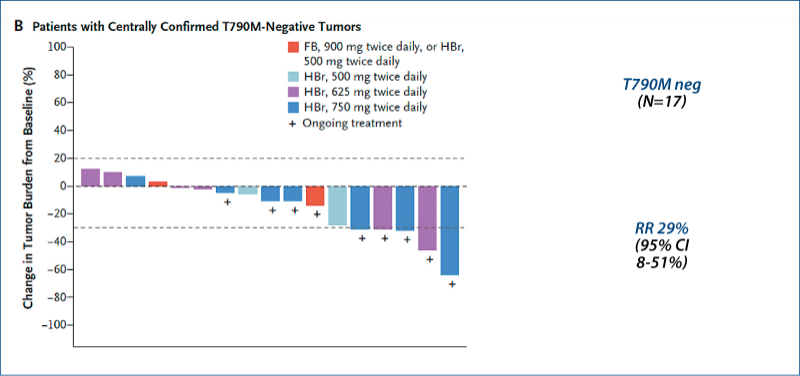
Rociletinib in EGFR-mutated NSCLC
(Sequist LV et al. NEJM, 2015, 372, 1700)
Dose-limiting toxicities were <33% at all dose levels.
Adverse events (all grades)
Hyperglycemia 36%
Nausea 31%
Fatigue 24%
Diarrhea 20%
Decreased appetite 15%
Rash <1%
Hyperglycemia was well managed with oral hypoglycemics and/or dose reduction.
Recommended phase II dose: 750 mg twice daily
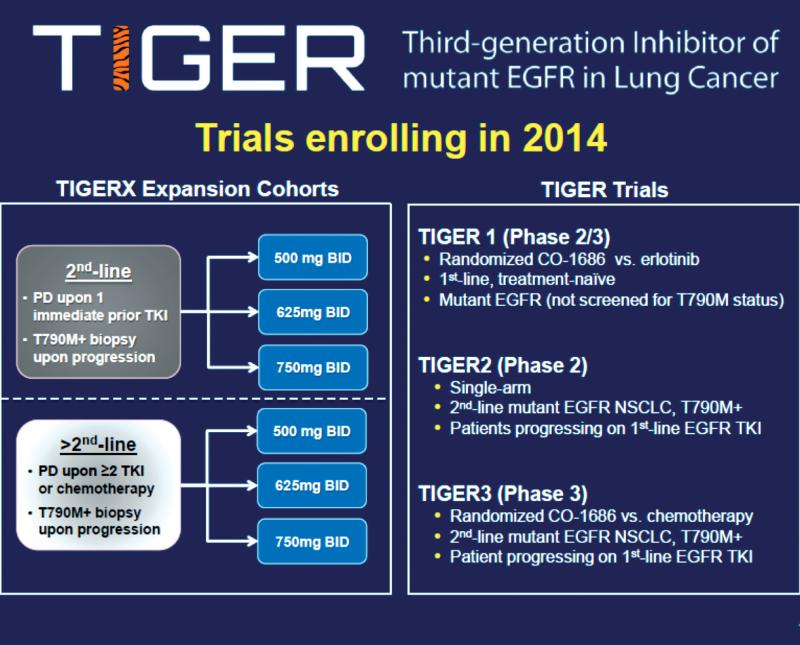
3rd generation EGFR tyrosine kinase inhibitors
Summary
Targeting EGFR-activating mutations and T790M mutation while sparing wild-type EGFR
Response rates around 60%
AZD9291
-
Phase III trials: AURA 3, FLAURA, ADAURA
-
ASTRA: Early access program
Rociletinib (CO-1686)
-
TIGER trials
HM61713
Crizotinib in advanced NSCLC
EML4-ALK-positive NSCLC
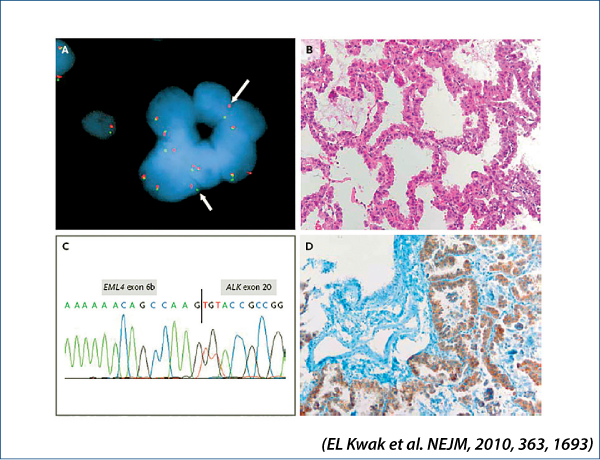
Crizotinib versus chemotherapy in advanced ALK-positive NSCLC: Profile 1007
(Shaw A et al. NEJM, 2013, 368, 2385)
Patients (n=347) pre-treated with chemotherapy
Crizotinib docetaxel or pemetrexed
Response rate 65% 20%
PFS median 7.7 months 3 months
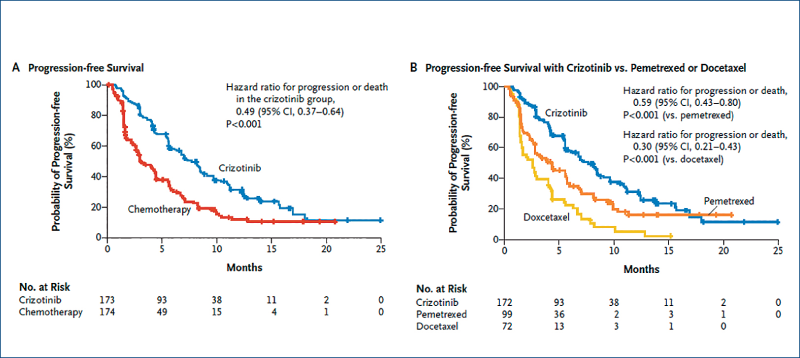
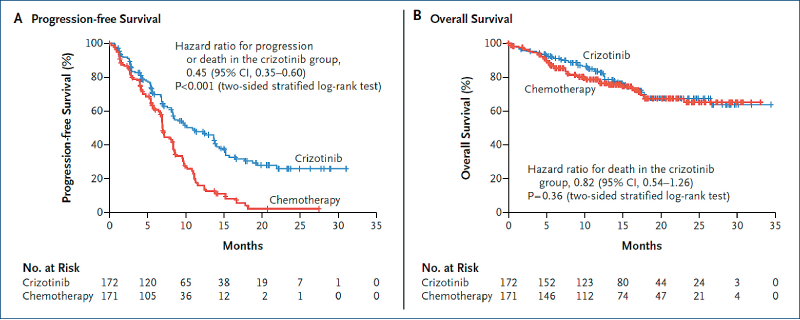
First-line crizotinib versus chemotherapyin ALK-positive NSCLC: Profile 1014
(Solomon BJ et al. NEJM, 2014, 371, 2167)
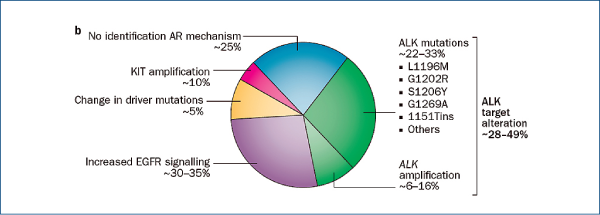
Crizotinib-resistant NSCLC
(Camidge R et al. Nat Rev Clin Oncol, 2014, 11, 473)
Ceritinib in ALK-rearranged NSCLC
(Shaw A et al. NEJM, 2014, 370, 1189)
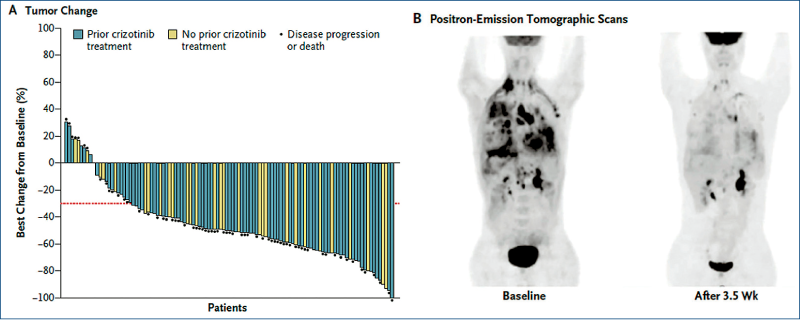
Advanced NSCLC
Palliative 1st line chemotherapy
Platinum-based doublets with 3rd generation drug
(vinorelbine, gemcitabine, paclitaxel, docetaxel, pemetrexed, nab-paclitaxel); 4-6 cycles
Symptom relief in approximately 50-60%
1-year survival rate increased by absolute ~10%
Improvement of quality of life (?)
(NSCLC Collaborative Group. BMJ 1995;311:899-909
NSCLC Meta-Analyses Collaborative Group. J CO 2008;26:4617-25)
Performance status affects outcome.
Elderly patients and patients with poor PS also benefit.
-
Well tolerated protocols
-
Enhanced supportive care
Chemotherapy of advanced NSCLC
Recent developments
Novel cytotoxic drugs
Customized chemotherapy based on biomarkers
Changes in administration and/or schedule
-
Oral administration
-
Chronic administration of low doses at regular intervals (metronomic chemotherapy)
Combination with targeted therapies
-
Angiogenesis inhibitors
Bevacizumab
Ramucirumab
Nintedanib
-
Anti-EGFR monoclonal antibodies
Cetuximab
Necitumumab
Bevacizumab in advanced non-squamous NSCLC
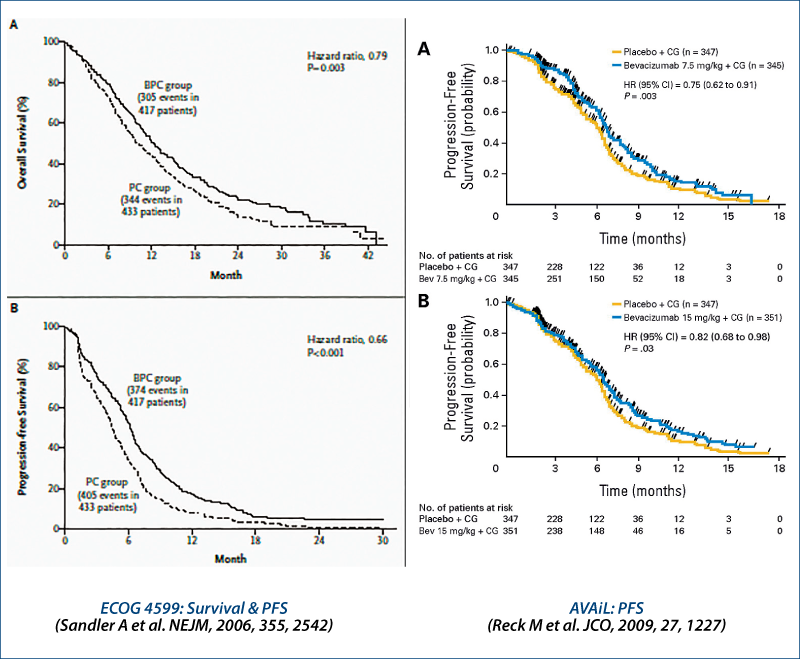
1st line chemotherapy ± EGFR antibodies: survival
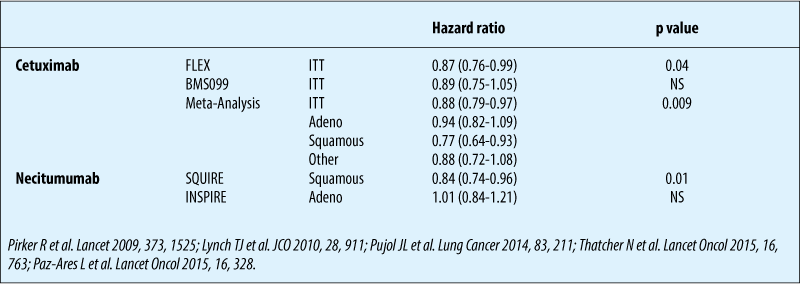
1st line chemo ± necitumumab in squamous NSCLC - overall survival
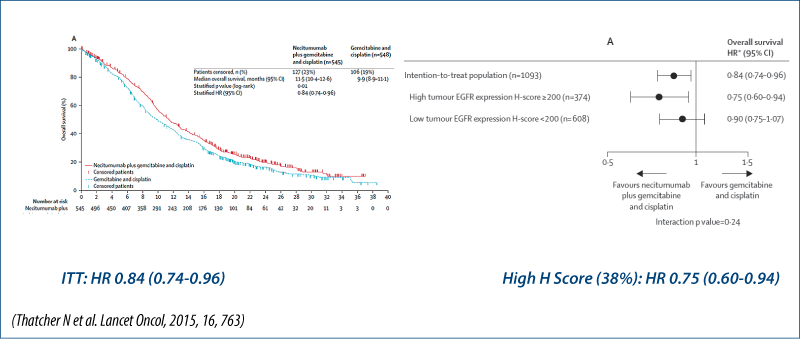
Survival based on biomarkers
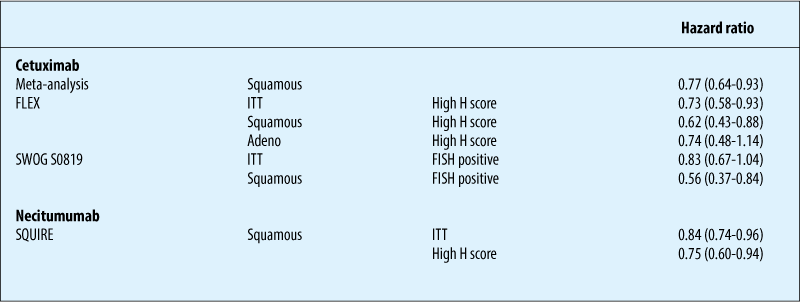
Chemotherapy ± anti-EGFR antibodies: biomarkers
Summary
Maintenance therapy: Phase III trials
Vinorelbine (Westeel V et al. JCNI, 2005, 97, 499)
Gemcitabine
-
CECOG (Brodowicz T et al. Lung Cancer, 2006, 52, 155)
-
US-Studie (Belani CP et al. JCO, 2010, 28, 15s (Abstr 7506))
-
IFCT-GFPC (Perol M et al. JCO, 2012, 30, 35160)
Pemetrexed
-
JMEN (Ciuleanu T et al. Lancet, 2010, 374, 1432)
-
PARAMOUNT (Paz-Ares L et al. Lancet Oncol, 2012, 13, 247)
-
AVAPERL (Barlesi F et al. JCO, 2013, 31, 3004 & Ann Oncol, 2014, 25, 1044)
Erlotinib
-
SATURN (Cappuzzo F et al. Lancet Oncol, 2010, 11, 521)
-
IFCT-GFPC (Perol M et al. JCO, 2012, 30, 3516)
Beva + Erlo (ATLAS) (Johnson BE et al. JCO, 2013, 31, 3926)
Gefitinib (INFORM) (Zhang L et al. Lancet Oncol, 2012, 13, 466)
Palliative therapy in pre-treated patients
Docetaxel
(Shepherd FA et al. JCO, 2000, 18, 2095; Fossella FV et al. JCO 2000, 18, 2354)
Pemetrexed (non-squamous NSCLC)
(Hanna N et al. JCO, 2004, 22, 1589)
Erlotinib
(Shepherd FA et al. NEJM, 2005, 353, 123)
Crizotinib in ALK-positive tumors
(Shaw A et al. NEJM, 2013, 368, 2385)
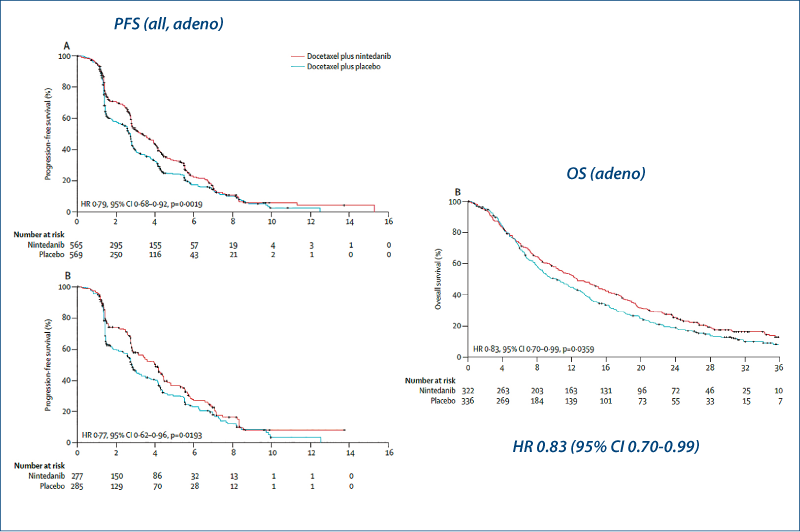
Docetaxel plus nintedanib
(Reck M et al. Lancet Oncol, 2014, 15, 143)
Docetaxel plus ramucirumab
(Garon EB et al. Lancet, 2014, 384, 665)
Afatinib in squamous cell NSCLC
(Soria J-C et al. Lancet Oncol, online 6 July 2015)
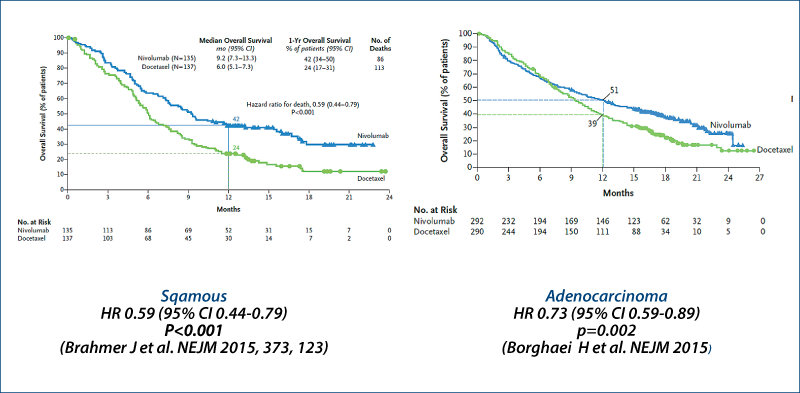
Nivolumab
(Brahmer J et al. NEJM, 2015, 373, 123; Borghaei H et al. NEJM, online 27 Sept 2015)
Docetaxel ± nintedanib: LUME-Lung 1
(Reck M et al. Lancet Oncol, 2014, 15, 143)
Docetaxel ± ramucirumab: REVEL
(Garon EB et al. Lancet 2014, 384, 665)

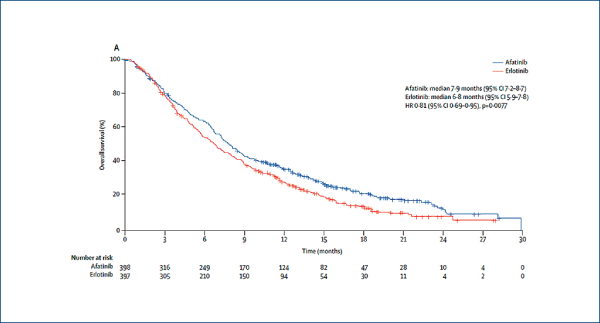
Afatinib versus erlotinib in squamous cell carcinoma of the lung: LUX-Lung 8
(Soria J-C et al. Lancet Oncol, 2015, online July 6)
Immune checkpoint inhibitors in advanced NSCLC
(Helissey C et al. Curr Opin Oncol, 2015, 27, 108)
Anti-CTLA4
-
Ipilimumab
-
Tremelimumab
Anti-PD-1
-
Nivolumab
-
Pembrolizumab
Anti-PD-L1
-
BMS-936559
-
Atezolizumab (MPDL3280A)
-
Tremelimumab (MEDI4736)
-
Avelumab (MSB0010718C)
Others
Nivolumab vs. docetaxel in advanced NSCLC Survival
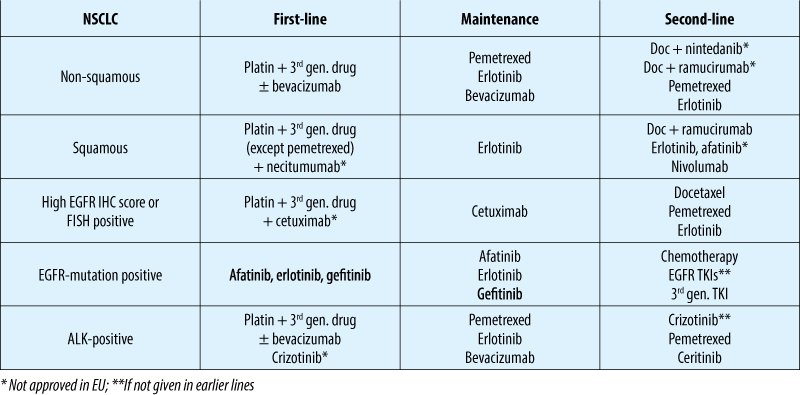
Advanced NSCLC: treatment options (10/2015)