Human papillomaviruses (HPV) are small icosahedral DNA non-enveloped viruses, adapted to human beings from the very beginning, since the apparition of the first humanoids. They are part of the large papillomaviruses group, which co-evolved with the first reptile species, around 350 million years ago. These viruses persist for years and even decades together with their animal or human hosts, in epithelial cells and/or mucosae, being considered rather commensal or producing chronic unapparent “infections” in most cases. There are described more than 150 HPV species, included in Alpha, Beta, Gamma, and some in Miu and Niu genera. The mechanisms of co-evolution between the human host and HPV are complex and depend on the host immune tolerance (with unknown advantages from HPV, to be tolerated) and HPV replication, with the promotion of host epithelial cell persistence, which in the case of HPV species with malignant risk could cause an “immortality” of the host-cell and consequently malignancy. The mucosal-associated HPV lesions, condyloma acuminatum and focal epithelial hyperplasia, are benign lesions caused by types of HPV with mucosal affinities. Other lesions are malignant, such as cervical or other anogenital cancers (of vulva, vagina, penis and anus) and head and neck cancers. The HPV species associated with cancers are included mostly in Alpha HPV genus. Cutaneous associated HPV lesions – common warts, plantar warts, flat warts, filiform warts, pigmented warts, epidermoid cysts – are benign lesions associated with cutaneous HPV strains, but Bowen disease is a type of skin cancer associated with alpha HPV. There are also suggestions that some of non-melanoma skin cancers could be associated with some beta HPVs. Curative measures include topical cytotoxic and antiviral agents, topical immunomodulatory agents, intralesional immunotherapy, destructive methods and surgery. Vaccination is the only way to decrease the incidence of HPV-induced cancers and should be indicated for both boys and girls before natural infection occurs.
HPV – schiţă a stilului său de viaţă
HPV sketch of its lifestyle
First published: 09 aprilie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ORL.51.2.2021.4948
Abstract
Rezumat
Papilomavirusurile umane (HPV) sunt virusuri mici neînvelite cu ADN icosaedric, adaptate fiinţelor umane chiar de la început, de la apariţia primilor umanoizi. Acestea fac parte din grupul mare de papilomavirusuri, care a coevoluat cu primele specii de reptile, cu aproape 350 de milioane de ani în urmă. Aceste virusuri persistă ani şi chiar decenii împreună cu gazdele lor animale sau umane, în celulele epiteliale
şi/sau mucoase, fiind considerate mai degrabă comensale sau producând „infecţii” cronice inaparente în majoritatea cazurilor. Sunt descrise mai mult de 150 de specii HPV, incluse în genurile Alfa, Beta, Gama şi unele din genurile Miu şi Niu. Mecanismele de coevoluţie între gazda umană şi HPV sunt complexe şi depind de toleranţa imună a gazdei (cu avantaje necunoscute de la HPV, pentru a fi tolerate) şi de replicarea HPV, cu promovarea persistenţei celulei-gazdă epiteliale, care, în cazul speciilor de HPV cu risc malign, ar putea provoca o „nemurire” a celulei-gazdă şi în consecinţă malignitate. Leziunile mucoase asociate HPV – condiloamele acuminate şi hiperplazia epitelială localizată – sunt leziuni benigne cauzate de speciile de HPV cu afinitate pentru mucoase. Alte leziuni sunt maligne, cum ar fi cancerul de col uterin sau alte tipuri de cancer anogenital (al vulvei, vaginului, de penis şi anus) şi cancerul din sfera cap şi gât. Speciile de HPV asociate cu cancerele sunt incluse mai ales în genul alfa. Leziunile cutanate asociate HPV – negi comuni, negi plantari, negi plaţi, negi filiformi, negi pigmentaţi, chisturi epidermoidale – sunt leziuni benigne asociate cu tulpini cutanate de HPV, dar boala Bowen este un tip de cancer de piele asociat cu alfa HPV. Există, de asemenea, sugestii că unele dintre cancerele de piele non-melanom ar putea fi asociate cu unele tipuri de beta HPV. Măsurile curative includ agenţi citotoxici şi antivirali topici, agenţi imunomodulatori topici, imunoterapie intralezională, metode distructive şi chirurgia. Vaccinarea este singura modalitate de scădere a incidenţei cancerelor induse de HPV şi ar trebui să fie indicată atât pentru băieţi, cât şi pentru fete, înainte ca infecţia naturală să se dezvolte.
Natural evolution of Papillomaviridae
Research suggests that the contemporary types of papillomaviruses evolved from ancestral variants which colonized the skin of the first reptiles around 350 million years ago. Through a continuous process of adaptation, nowadays papillomaviruses are found on the epithelial surface of a large number of species, including humans. These viruses have a high species specificity and, like humans, mammals have their own papillomaviruses(1) (Figure 1).
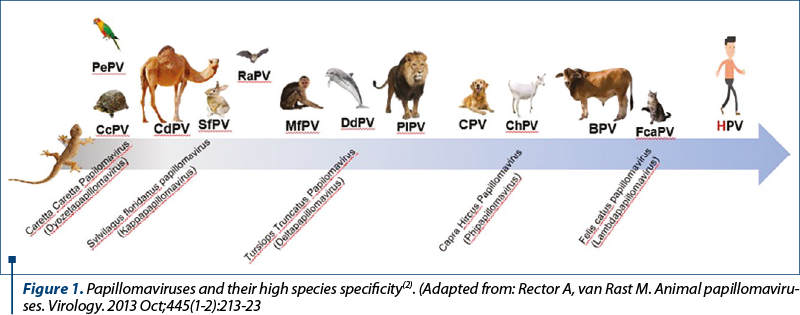
Initially, Papillomavirus and Polyomavirus genera were part of the Papovaviridae family that later was divided into two distinct families based on genome structural differences. Despite both genera are non-enveloped DNA viruses with oncogenic properties, they are distinct in term of genome size, organization and sequence(3).
Papillomaviridae family contains more than 150 types of viruses with human tropism – human papillomavirus (HPV). In most cases, HPV can be considered as a commensal due to the evidence of HPV DNA positivity on the skin of healthy individuals(1).
Clinical aspects of HPV infections:
history and present
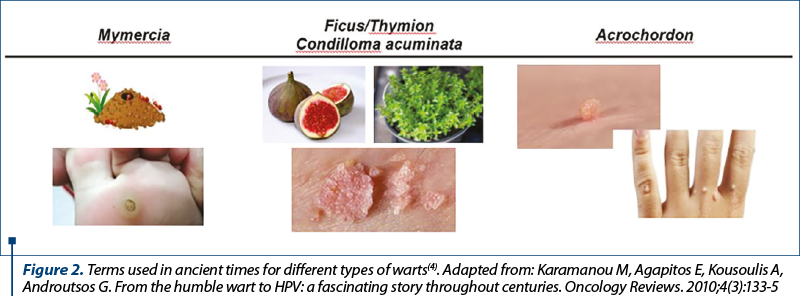
It seems that our ancestors were also preoccupied about HPV-induced lesions. Ever since Hippocrates’s time, this pathology was described based on the similarities between the visual appearance of lesions and other elements found in nature.
Therefore, mymercia was the term used for plantar warts because of its resemblance with an ant nest; thymion was the name given for anogenital warts which resemble with thyme leaves, or ficus due to its resemblance with the core of a fig. Acrochordon was the name given to common warts because they appear on the extremities and resemble a string. The current term used for anogenital warts, condyloma acuminatum, was used since ancient times and it was associated with promiscuity(4) (Figure 2).
The majority of HPV types cause benign and self-limiting lesions of the epithelial tissue. Common warts, plantar warts, flat warts, filiform warts and pigmented warts are frequently seen in children and young people, HPV 1, HPV 2, HPV 4, HPV 27 and HPV 57 being the most common strains involved. Compared to healthy people, where spontaneous resolution is commonly seen, in immunosuppressed patients, the HPV lesions often do not resolve without treatment and recurrences are frequently seen(1).
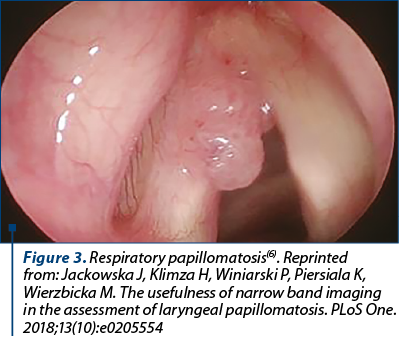
Condyloma acuminatum is the most common sexually transmitted infectious disease, HPV 6 and HPV 11 being responsible for the majority of cases. These strains have a low oncogenic risk, producing benign lesions in the vast majority of cases. The issues arise from the fact that these lesions are difficult to treat and these HPV types can be vertically transmitted during delivery, leading to recurrent respiratory papillomatosis (RRP) in newborns. RRP is a debilitating disease which can lead to airways obstruction, surgical excision being the only treatment option. However, this disease tends to relapse or extend to lower respiratory and digestive tracts, with the possibility of progression to carcinoma(1,5) (Figure 3).
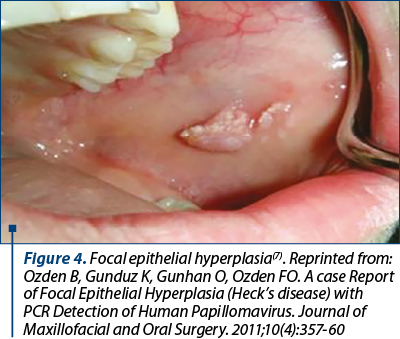
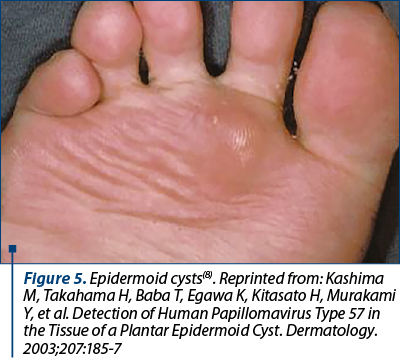
Focal epithelial hyperplasia and epidermoid cysts are other types of HPV-related lesions caused by HPV 13 and 32 and by HPV 57 and 60, respectively(1) (Figure 4 and 5).
HPV structure and classification
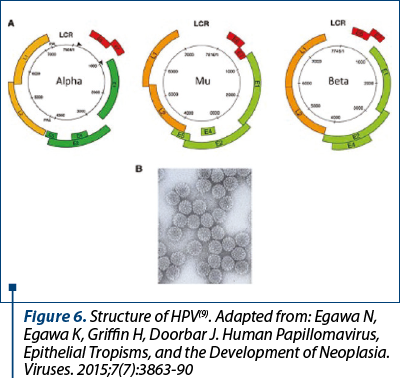
In terms of structure, HPV is a small DNA non-enveloped virus. The genome is shielded by an icosahedral capsid composed of 72 capsomeres, and encodes three regions: “early”, “late” and “long control region”. The early region comprises six genes (E1, E2, E4, E5, E6 and E7) which encode proteins with roles in: viral replication, release of new mature virions, structural changes of the keratinocyte with the apparition of the koilocyte (the hallmark of HPV infection), and eventually cell immortalization, with the possibility of malignization. E6 and E7 are the two major proteins which confer HPV carcinogenic properties. The late region is composed by two genes (L1 and L2) which encode the capsid proteins with roles in viral assembly and cell entry. Late proteins have also immunogenic properties. The long control region has a role in regulation of viral replication(9) (Figure 6).
HPVs are classified in five genera: Alpha (a), Beta (b), Gamma (g), Miu (µ) and Niu (n) – Figure 7.
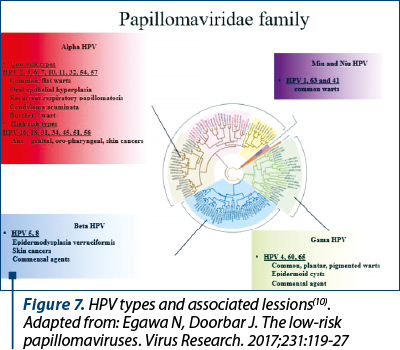
Alpha papillomavirus is by far the most important genera because it includes strains with high oncogenic risk. HPV 16 and HPV 18 are known as the main etiological factors of cervical carcinomas, also having an important role in the etiopathogenesis of other anogenital malignancies, such as anal, vulvar, vaginal and penile cancers(1).
In recent years, it has been proven that HPV also plays a crucial role in head and neck carcinomas, HPV 16 and 18 being the most involved. Interestingly, HPV 6 and 11, which are considered low-risk oncogenic types, commonly causing benign anogenital lesions, have also been detected in patients with oropharyngeal non-squamous cell carcinoma. This observation reinforces the theory that even low-risk oncogenic types of HPV may give rise to malignancies. Nowadays, HPV-related oropharyngeal cancers are responsible for 70% of cases, therefore it is now being regarded as a separate clinical entity with increasing incidence and tendency to become the main HPV-related cancer(11-13).
Some types of HPV are also considered to cause some types of skin malignancies. It seems that beta papillomaviruses are the main types found in non-melanoma skin cancer biopsy samples, followed by the Alpha genera. Beta HPV 5 and 8 are the main strains encountered in squamous cell carcinoma of patients with epidermodysplasia verruciformis, a rare autosomal recessive disease, leaving the person defenseless against the HPV infection. Bowen’s disease, a squamous cell carcinoma in situ, can progress to an invasive form and seems to be associated more frequently with alpha HPV 16(14,15).
Gamma, Miu and Niu genera are less involved in human pathology, causing in general benign lesions such as common warts(10).
Pathophysiology. From infection
to malignancy
HPV is an epitheliotropic virus that infects the basal layer of the epithelium via microwounds of the skin and mucosae. At this level, HPV enters the cells through a6-integrin and syndecan-1 receptors. After binding, the virus is internalized into the cell by endocytosis. The basal layer of the epithelium is composed by stem cells which are capable of division, thus maintaining the integrity and ensuring healing in case of epithelial injuries. After cell entry, viral genome is integrated into basal cells and viral replication is maintained, but with a low number of copies. Following stem cell division, the viral genetic material is transmitted to each daughter cell which will further differentiate into keratinocytes. This process is essential for viral maturation because the formation of mature virions, capable of producing a new infection, is possible only in the superficial layers(1,16,17). At this level, within the practically apoptotic cells, which are losing their mitochondria, HPV encounters an oxidizing environment necessary for its assemblance(1). After complete maturation, these viral particles are released from keratinocytes during the process of physiologic desquamation. Therefore, if these new virions encounter another microwound, they will start a new cycle of infection(16,17).
The incubation period for HPV varies widely from a few weeks to several years. In the great majority of cases, spontaneous clearance is seen before lesions occur via adaptive T-cell immune response between 6 month and 2 years, with a longer period for high-risk types of HPV(18,19).
Persistent infection carries the risk of developing malignancies. High-risk types of HPV have the ability of immune evasion based on their exclusively intraepithelial life cycle without producing cellular lysis, inflammation or viraemia. HPV can also downregulate cytokine release. The immunogenic viral particles are removed from the epithelial surface simultaneously with the normal process of desquamation, thus low viral levels are exposed to immune cells. Furthermore, weak recognition of viral particles by the antigen presenting cells delays the adaptive T-cell and humoral mediated immune response(20).
The key steps for carcinogenesis are: the integration of viral DNA in the host cell genome, inhibition of tumor suppressors like p53 and retinoblastoma protein (pRb), and the stimulation of the telomerase activity. All these processes are mediated by E6 and E7 oncoproteins, causing chromosomal abnormalities and immortalization of the abnormal infected cells, leading to the development of varying degrees of dysplasia and, finally, to neoplasia(20,21).
Viral transmission and measures to stop it
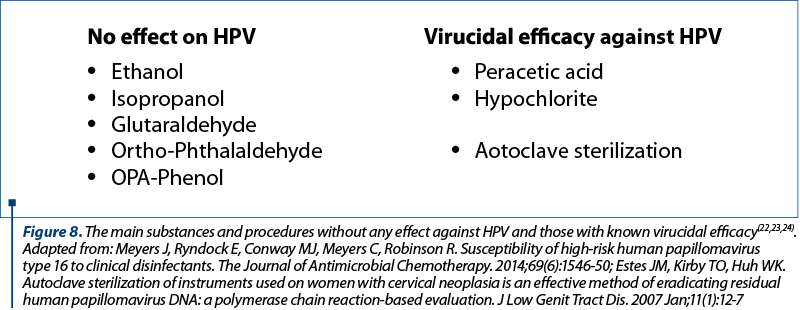
The most common mode of transmission is by sexual contact, HPV infection being one of the leading sexually transmitted diseases worldwide. The vertical transmission during delivery is also possible through the direct contact between mother’s infected mucosae and the newborn’s epithelial tissue. The possibility of transmission through direct skin contact, autoinoculation between anatomic sites and fomites, has also been suspected. Nosocomial transmission was also speculated due to the persistence of HPV DNA on gynecological instruments after disinfection. This emphasizes the necessity of knowing which types of substances or procedures have virucidal effect on HPV. Figure 8 shows the main substances and procedures with well-known virucidal efficacy and those without any effect against HPV(22-24).
Treatment. From Chelidonium majus
to contemporary management
It seems that the lesions induced by HPV also preoccupied our ancestors and they noticed that Chelidonium majus – or greater celandine – can lead to the resolution of common warts. They used the substance found in the stalk of the plant for topical application on the lesions. Later it was proven that the substance has antiviral, immunomodulatory and cytotoxic effects through the sanguinarine, a compound that blocks cell cycle and chelidonine which induces apoptosis through the stimulation of p53 and inhibition of telomerase activity. Nowadays, greater celandine is not approved for medical uses due to its systemic toxicity, even though there is no proof of systemic absorption after topical use. Indol-3-carbinol is another compound found in cruciferous that seems to have antiviral and antiproliferative activity(25-27).
The current treatment options for HPV-induced benign lesions include topical cytotoxic and antiviral agents (podophyllotoxin, cidofovir), topical immunomodulatory agents (imiquimod, sinecatechins, intralesional interferon, intralesional immunotherapy with mumps, Candida and Trichophyton skin test antigens) and destructive methods (trichloroacetic acid, cryotherapy, surgical or laser excision). Malignant lesions require surgical procedures(28).
An important prophylactic measure is to maintain the integrity of skin and mucosae, so that HPV would not reach the basal layer, the only site where it finds the receptors needed for binding and for entering the cell. These can be achieved through maintaining our skin hydrated and by avoiding microtrauma.
Because HPV infections which lead to malignant lesions are asymptomatic and the cancers are frequently diagnosed in late stages, it is of paramount importance to get preexposure prophylaxis. Vaccination is the only way to prevent the development of HPV-induced malignancies and also offers protection against HPV types that cause condyloma acuminatum. The current approved vaccine, Gardasil 9®, offers protection against nine of the most frequently involved strains: HPV 6, HPV 11, HPV 16, HPV 18, HPV 31, HPV 33, HPV 45, HPV 52 and HPV 58. The efficacy in preventing HPV infection is nearly 100%. The protection is achieved through a humoral immune response characterized by the production of neutralizing antibodies (IgG) which confer protection for at least 10 years. The humoral immune response after natural infection is weak, delayed and half of the patients don’t develop antibodies. Conversely, after vaccination, a robust response is seen, with faster seroconversion. This happens due to highly immunogenic virus-like proteins (VLPs) of the vaccine and the administration route. VLPs are purified L1 major capsid viral proteins that are highly immunogenic and have the property to self-assemble. The intramuscular administration allows a better interaction between immunogenic particles and immune cells.
Currently, the indication for vaccination starts at 9 years old up to 45 years old for both males and females. Between 9 and 14 years old, a two-dose regimen is considered sufficient, but over 14 years old, a three-dose regimen is necessary for providing a protective immune response. The purpose of vaccination is to decrease the incidence of anogenital and oropharyngeal malignancies induced by HPV(29,30).
Speculative discussion
The mechanism of co-evolution between HPV and human host is complex. HPV belongs to normal skin microbiota. More than fifteen types of beta and gamma papillomaviruses seem to live on healthy human skin without causing any damage. In order to maintain an equilibrium, the human body has to develop a certain tolerance for HPV. In return, HPV has to confer some advantages. As a member of human skin microbiota (along with bacteria, archaea and other viruses), HPV confers protection against other bacteria, fungi or other more virulent viruses(31).
Taking into account that HPV’s E6 and E7 oncoproteins stimulate the telomerase activity(24), we can speculate that HPV may act like an antiaging compound through preventing apoptosis. This could be another advantage conferred by HPV, in the absence of malignization. In this regard, it is interesting that the replication cycle of HPV is blocked in less differentiated neoplastic cells. Therefore, we assume that the initiation of carcinogenesis is not beneficial for HPVs.
Conclusions
Papillomaviridae is a large family of small DNA viruses with ancient origins. They are found on the skin and mucosae of many species, including humans, and are characterized by high species specificity, each vertebrate having its own type of papillomavirus.
In the majority of cases, it can be considered as a commensal (with unclear advantages for the host). Most infections are clinically unapparent, with viral clearance produced via cellular mediated immunity, but the persistence of HPV can give rise to malignancies.
Vaccination is the only way to decrease the incidence of HPV-induced cancers and should be indicated before natural infection occurs.
Bibliografie
-
Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Reviews in Medical Virology. 2015;25 (Suppl 1):2-23.
-
Rector A, Van Ranst M. Animal papillomaviruses. Virology. 2013;445(Issues 1–2):213-223.
-
de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004; 324(1):17-27.
-
Karamanou M, Agapitos E, Kousoulis A, Androutsos G. From the humble wart to HPV: a fascinating story throughout centuries. Oncology Reviews. 2010;4(3):133-5.
-
Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. The Laryngoscope. 2008;118(7):1236-47.
-
Jackowska J, Klimza H, Winiarski P, Piersiala K, Wierzbicka M. The usefulness of narrow band imaging in the assessment of laryngeal papillomatosis. PLoS One. 2018;13(10):e0205554.
-
Ozden B, Gunduz K, Gunhan O, Ozden FO. A case Report of Focal Epithelial Hyperplasia (Heck’s disease) with PCR Detection of Human Papillomavirus. Journal of Maxillofacial and Oral Surgery. 2011;10(4):357-60.
-
Kashima M, Takahama H, Baba T, Egawa K, Kitasato H, Murakami Y, et al. Detection of Human Papillomavirus Type 57 in the Tissue of a Plantar Epidermoid Cyst. Dermatology. 2003;207:185-7.
-
Egawa N, Egawa K, Griffin H, Doorbar J. Human Papillomavirus, Epithelial Tropisms, and the Development of Neoplasia. Viruses. 2015;7(7):3863-90.
-
Egawa N, Doorbar J. The low-risk papillomaviruses. Virus Research. 2017;231:119-27.
-
Miller DL, Puricelli MD, Stack MS. Virology and molecular pathogenesis of HPV (human papillomavirus)-associated oropharyngeal squamos cell carcinoma. The Biochemical Journal. 2012;443(2):339-53.
-
CDC: Top HPV-Associated Cancer Is Now Oropharyngeal. Medscape. Aug 23, 2018.
-
Pytunia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated orophryngeal cancer. Oral Oncology. 2014;50(5):380-6.
-
Didona D, Paolino G, Bottoni U, Cantisani C. Non Melanoma Skin Cancer Pathogenesis. Overview. Biomedicines. 2018;6(1):6.
-
Handisurya A, Schellenbacher C, Kirnbauer R. Diseases caused by human papillomaviruses (HPV). JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2009;7(5):453-66.
-
Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiology and Molecular Biology Reviews: MMBR. 2004;68(2):362-72.
-
Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virology Journal. 2010;7(1):2.
-
Oriel JD. Natural History of genital warts. The British Journal of Veneral Diseases. 1971;47(1):1-17.
-
Molano M, Van den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. American Journal of Epidemiology. 2003;158(5):486-94.
-
Stanley MA. Epithelial cell response to infection with human papillomavirus. Clinical Microbiology Reviews. 2012;25(2):215-22.
-
Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Science. 2007;98(10):1505-11.
-
Sabeena S, Bhat P, Kamath V, Arunkumar G. Possible non-sexual modes of transmission of human papilloma virus. The Journal of Obstetrics and Gynaecology Research. 2017;43(3):429-35.
-
Meyers J, Ryndock E, Conway MJ, Meyers C, Robinson R. Susceptibility of high-risk human papillomavirus type 16 to clinical disinfectants. The Journal of Antimicrobial Chemotherapy. 2014;69(6):1546-50.
-
Estes JM, Kirby TO, Huh WK. Autoclave sterilization of instruments used on women with cervical neoplasia is an effective method of eradicating residual human papillomavirus DNA: a polymerase chain reaction-based evaluation.
-
J Low Genit Tract Dis. 2007 Jan;11(1):12-7.
-
Nawrot J, Wilk-Jędrusik M, Nawrot S, Nawrot K, Wilk B, Dawid-Pać R, et al. Milky Sap of Greater Celandine (Chelidonium majus L.) and Anti-Viral Properties. International Journal of Environmental Research and Public Health. 2020;17(5):1540.
-
Jin L, Qi M, Chen D-Z, Anderson A, Yang G-Y, Arbeit JM, et al. Indole-3-Carbinol Prevents Cervical Cancer in Human Papilloma Virus Type 16 (HPV 16) Transgenic Mice. Cancer Research. 1999;59(16):3991-7.
-
Singh AA, Patil MP, Kang M-J, Niyonizigiye I, Kim G-D. Biomedical application of Indole-3-carbinol: A mini-review. Phytochemistry Letters. 2021;41:49-54.
-
Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. The third efition. Volume one. 2012 Elsevier Limited; page 1314.
-
Bonanni P, Boccalini S, Bechini A. Efficacy, duration of immunity and cross protection after HPV vaccination: a review of the evidence. Vaccine. 2009;27 Suppl 1:A46-53.
-
Gardasil 9 [package insert]. Merck & Co., INC. 2006-2020. US Food and Drug Administration; website: https://www.fda.gov/media/90064/download
-
Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, et al. Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One. 2012;7(6):e38499.