In clinical practice, we are often confronted with dental postoperative pain that occurs after coronary restoration with resin composites. This article reviews the causes of sensitivity after the placement of a composite obturation, the pathological mechanisms, the involved mediators and the importance of differential diagnosis with dentinal pain from pulp inflammation. In this context, the role played by the marginal microleakage in the appearance of this dentinal pain is emphasized.
Sensibilitatea postoperatorie asociată restaurărilor cu răşini compozite
Postoperative sensitivity associated with resin composite restorations
First published: 30 noiembrie 2018
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Orl.41.4.2018.2121
Abstract
Rezumat
În practica clinică ne confruntăm adesea cu durerea postoperatorie dentinară care apare după restaurarea directă coronară cu răşini compozite. Articolul de faţă trece în revistă cauzele sensibilităţii după obturaţiile cu compozite, mecanismele patologice de apariţie, mediatorii implicaţi, precum şi importanţa diagnosticului difenţial cu durerea dentinară din inflamaţia pulpară. În acest context, este subliniat rolul pe care îl joacă microinfiltraţia marginală în apariţia acestei dureri dentinare.
Most dental materials allow marginal microleakage at the interface between the restoration and the dental tissue. This microleakage of oral fluids, containing bacteria and their products, can penetrate deep inside the dentin tissue.
Therefore, an understanding of the clinical consequences of microleakage requires, among other parameters, the analysis of the dentin permeability characteristics.
There is a balance between the diffusion of bacterial products that penetrate the dentin through microleakage and the rate at which they are removed by the pulp circulation mechanisms. Decreasing pulp blood flow allows the concentration of these products to increase, triggering pulp inflammation.
From clinical practice, we know that sometimes painful sensitivity occurs after composite resines restorations, but it is not produced by inflammatory dental pulp phenomena. It is well known that in dentistry, the reason patients get into the dental practice is often dental pain.
If composite materials would seal dentin as the enamel and cement do, there would be no detectable microleakage, and therefore no clinical problems associated with their use.
Microleakage is a clinical serious problem, requiring a detailed analysis and discussion because most composite resins direct restorations exhibit different degrees of microleakage.
Patients sometimes complain of dental sensitivity or pain immediately after the composite is placed into cavity. This is dentinal pain that can be given by an exposed dentin area which is not covered by the dental restorative material. But most often, dentinal pain occurs as a result of microleakage between dentin and composite resin. This distinct and well-located pain indicates direct contact and transfer between pulp and oral fluids. This means there is a direct communication between the oral environment and the pulp, but in our case other than the accidental opening of the pulp chamber.
Frequently, this occurs along the gingival margin in class II restorations because of the polymerisation shrinkage of a composite resin, leading to marginal failure and a fluid-filled space (Figure 1 and Figure 2).
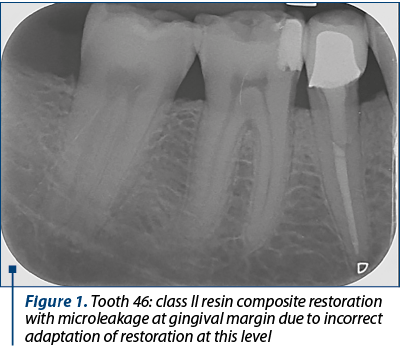
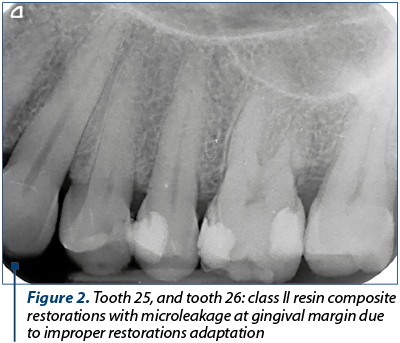
Composite resins have a modulus of elasticity that allows them to flex under the action of occlusal forces. Therefore, in a sense, the sudden, well-located and transient pain due to the sensitivity of the dentin is positive, helping us in the differential diagnosis of dental sensitivity of a healthy pulp versus dental pulp inflammation.
Dentinal pain occurs due to fluid movements within open dentinal tubules to the pulp where the A delta nerve fibers are activated (they serve as mechano-receptors), so an outside stimulus is felt as pain. The A delta myelinated fibers are fast-conducting intradental nerves, with high diameter and low stimulation threshold. They can be stimulated without tissue damage and are superficially located. The A delta fibers react even to mechanical, thermal and osmotic smooth agents, and are involved only in the initial phases of inflammation.
Dentinal sensitivity depends on its degree of permeability:
-
Thin dentin is more permeable than thicker dentin.
-
The dentin that coats the pulp horns is more permeable than the one near the ceiling of the pulp chamber.
-
The dentine that forms the axial walls is more permeable than the one forming the cavity floor.
-
Coronary dentin is much more permeable than root dentine.
Another extremely important thing about the dentine permeability is the presence or absence of smear layer, as a result of the dentin excavation process.
The smear layer is an amorphous film composed of hydroxyapatite and altered collagen. Inside every dentinal tubules the smear layer enters on different depth between 4.5-8.6 µ, forming “smear plugs”. The smear plugs reduce the permeability of the dentin more than the smear layer that covers them.
Smear layer is a natural liner that reduces dentinal permeability much better than any dental material does. However, its presence limits the value of the dentinal sealing and adhesion due to the low cohesion forces between it and the dentin.
If the smear layer is removed, the value of dentinal bonding increases, but also the chances of pulp inflammation if the adhesive technique is not perfectly applied.
Most current studies have concluded that a small infiltration always occurs in the absence of smear layer. Brannstrom (1984) claims to remove smear layer present on dentinal prepared surfaces using a very diluted solution of 0.2% ethylenediaminetetraacetic acid (EDTA). This will ensure a better sealing of the composite resin to the hard dental structure without removing the dentin chips which helps to reduce dentinal permeability.
The latest generations of dentin adhesives use acid etching or chelation. Properly used, these adhesives completely remove residual dentine debris and increase adhesion of resin composites, but also increase dentinal permeability.
Marginal microleakage is amplified by the accumulation of dental bacterial plaque. The hydrostatic pulp pressure is greater than that of the fluids in the oral cavity. That is why the dentinal fluid circulates through the dentinal tubules and probably through all the microleakage spaces and gaps in the opposite direction to the movement of the bacterial products. High molecular weight proteins such as fibrinogen may reduce the permeability of dentin by their absorption into the dentinal tubules walls.
In this context, let us see how the dentinal sensitivity resulting from microleakage develops in dental restorations using resin composites.
The persistence of the bacterial irritants diffusion through dentin leads to the activation of C nerve fibers which contribute to the inflammatory process by releasing neuropeptides.
Today, a number of neuropeptides that act as neurotransmitters and neuromodulators are known in the dental pulp. These include: P substance, CGRP (Calcitonin Gene Related Peptide), neurokinin A (NKA), neuropeptide Y (NPY).
Substance P is the first neuropeptide that has been proposed as a neurotransmitter in the dental pulp. P substance interacts with blood vessels, causing vasodilatation and plasma extravasation, as well as profound inflammatory and immune response.
Neuropeptide P-containing nerve fibers are generally observed around the blood vessels, then penetrating into the predentin area with different distribution patterns.
Nerve fibers containing CGRP are uniformly distributed in the pulp, penetrating as nerve fibers with substance P also predentin and dentin. Nerve fibers with CGRP play a major role in neurogenic inflammation, and are therefore important in dental pulpal pain and pathology.
Neurokinin A is one of the most recently neuropeptides discovered in the dental pulp. It now seems like these neuropeptides are contained in the same nerve fibers.
In addition to this, neuropeptide Y has been found, which appears in the structure of sympathetic nerve fibers, and neuropeptide VIP (vasoactive intestinal polypeptide).
Nerve fibers that contain neuropeptides and are associated with blood vessels play an important role in changes in pulp blood flow.
The discovery of the neuropeptides involved in pulp inflammation has revolutionized the concepts so far. Today, it is clear that neuropeptides present in some pulp nerve fibers play an important role in the progression of pulp inflammation by combining sensitive activity and microcirculation.
These are the mechanisms and the substances that can lead to a pulp inflammation after the placement of a direct composite resin restoration, even if the pulp protection agents and bases are correctly used.
Concluding, we can say that it is important to know how to differentiate the dentinal sensitivity from the dental pulp inflammation pain. But it is also very important to be aware that continuous marginal microleakage, by stimulation of receptors and nerve fibers, triggers inflammatory phenomena.
Acknowledgements: For this article, all the authors have equal contributions.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Vârlan C, Dimitriu B, Stanciu D, Suciu I, Chirilă L. Situaţia actuală a adeziunii la structurile dure dentare. SSER - Societatea de Stomatologie Estetică din Romania, 2011; Bucureşti, pp. 8-10, 16-22.
- Cortes O, Alcaina A, Bernabe A. Biocompatibility Evaluation of Four Dentin Adhesives Used as Indirect Pulp Capping Materials. Acta. Stomatol. Croat. 2017; 51(2): 113–121.
- Gheorghiu IM, Mărăscu V, Staicu D, Zmarandache D, Perlea P. Comparative ultrastructural analysis of dentin surfaces mechanically prepared using carbon steel conventional burs and polymer burs. Rom. Biotechnol. Lett. 2017; 22(4): 12775-12784.
- West NX, Lussi A, Seong J, Hellwig E. Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig. 2013; 17(Suppl 1): 9–19.
- Brännstrom M. Smear layer - pathological and treatment considerations. Oper. Dent. 1984; Supplement 3: 35-42.
- Närhi M. The neurophysiology of the teeth. Dent Clin North Am. 1990; 34:439–448.
- Estay J, Martin J, Vildósola P, Villablanca C, Mjör I, de Andrade MF, Moncada G, Gordan VV, Opdam N, Fernández E. Sealing of restorations with marginal defects does not affect their longevity. Am J Dent. Apr 2018; 31(2):107-112.
- Scotti N, Bergantin E, Giovannini R, Delbosco L, Breschi L, Migliaretti G, Pasqualini D, Berutti E. Influence of multi-step etch-and-rinse versus self-etch adhesive systems on the post-operative sensitivity in medium-depth carious lesions: An in vivo study. Am J Dent. Aug 2015; 28(4):214-8.
- Perdigão J, Swift EJ. Critical appraisal: post-op sensitivity with direct composite restorations. J Esthet Restor Dent. Aug 2013; 25(4):284-8.
- Umer F, Khan FR. Postoperative sensitivity in Class V composite restorations: Comparing soft start vs. constant curing modes of LED. J Conserv Dent. Jan 2011; 14(1):76-9.
- Larson TD. The clinical significance and management of microleakage. Part one. Northwest Dent. Jan-Feb 2005; 84(1):23-5, 28-9.
Articole din ediţiile anterioare
Restaurarea directă în zona frontală a distrucţiilor dentare coronare – prezentare de caz
Acest articol prezintă o temă de actualitate în domeniul medicinei dentare restaurative: restaurarea directă a distrucţiilor dentare coronare în...
Esthetic function direct restoration in the anterior area – case report
Restaurarea cu succes a funcţiei fizionomice a zonei anterioare a arcadelor dentare impune ca medicul dentist să aibă cunoştinţe avansate despre ...
Tratamentul leziunilor dentare necarioase
Leziunile dentare necarioase – fie că sunt cu sau fără pierdere de substanţă – conduc la apariţia tulburărilor fizionomice, având, de asemenea, un ...
Direct composite veneers for erosive-abrasive lesions – case report
Tratamentul leziunilor dentare abrazive şi/sau erozive este unul conservator. Una dintre metodele de tratament este faţetarea directă sau indire...