Vesicoureteral reflux is the most common urinary tract abnormality among children, estimated to be present in approximately 1-2% of them. There are various possibilities of evolution, from asymptomatic forms with spontaneous remission to recurrent acute pyelonephritis, reflux nephropathy, or even end-stage chronic kidney disease. Therefore, the early diagnosis, with a correct staging, is essential, allowing the optimal therapeutic approach. The most common clinical situations requiring imaging investigation of vesicoureteral reflux are repeated urinary tract infections, fetal hydronephrosis, familial vesicoureteral reflux, bladder bowel dysfunction, and complex uropathies. The therapeutic management is decided according to the reflux severity, the clinical manifestations, and the patient’s age. The main objectives of the treatment are to prevent urinary tract infections, the development of renal scars, and the progression toward chronic kidney disease. The therapeutic approach may involve active follow-up, continuous antibiotic prophylaxis, or surgical intervention. Active follow-up applies to asymptomatic patients with an increased likelihood of spontaneous remission. Antibiotic prophylaxis is the most frequently adopted approach, used in patients with previous urinary tract infections and high-grade reflux. The surgical approach is reserved for cases with a low probability of spontaneous remission and for those with unfavorable evolution under conservative treatment. The surgical methods used are represented by endoscopic injection of an antireflux substance, classical or robotically assisted surgery, with similar success rates. Currently, there is no consensus regarding the therapeutic approach to these patients, the pediatrician being the one who must evaluate each case and propose and individualized therapeutic management adapted to the needs of each patient.
Clinical approach to children with vesicoureteral reflux – an overview
Abordarea clinică a copiilor cu reflux vezico-ureteral – prezentare generală
First published: 30 aprilie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.69.1.2023.7980
Abstract
Rezumat
Refluxul vezico-ureteral reprezintă cea mai frecventă anomalie a tractului urinar în rândul copiilor, estimându-se a fi prezent la aproximativ 1-2% dintre aceştia. Posibilităţile evolutive sunt extrem de variate, de la forme asimptomatice cu remisiune spontană la pielonefrite acute repetate, nefropatie de reflux sau chiar boală cronică renală în stadiu final. De aceea, diagnosticul precoce, cu o corectă stadializare a gradului de reflux, este esenţial, permiţând abordarea terapeutică optimă. Cele mai frecvente situaţii clinice care impun investigarea imagistică a refluxului vezico-ureteral sunt infecţiile urinare repetate, hidronefroza fetală, refluxul vezico-ureteral familial, disfuncţiile vezicale şi uropatiile complexe. În funcţie de severitatea refluxului, manifestările clinice şi de vârsta pacientului, se decide conduita terapeutică. Obiectivele terapeutice principale sunt reprezentate de profilaxia infecţiilor urinare, prevenirea dezvoltării cicatricelor renale şi evitarea evoluţiei către boala cronică renală. Abordarea terapeutică poate presupune monitorizare activă, profilaxie antibiotică sau intervenţie chirurgicală. Monitorizarea activă se aplică pacienţilor asimptomatici care prezintă o probabilitate crescută de remisiune spontană a refluxului. Profilaxia antibiotică este abordarea cel mai frecvent adoptată, fiind utilizată la pacienţii cu infecţii urinare în antecedente şi în cazul refluxului de grad mare. Abordarea chirurgicală este rezervată cazurilor cu probabilitate mică de remisiune spontană şi celor cu o evoluţie nefavorabilă sub tratament conservator. Metodele chirurgicale utilizate sunt reprezentate de injectarea endoscopică de substanţă antireflux, chirurgia clasică sau asistată robotic, cu rate similare de succes. Actualmente nu există un consens privind abordarea terapeutică a acestor pacienţi, medicul pediatru fiind cel care trebuie să evalueze fiecare caz în parte şi să propună o conduită terapeutică individualizată, adaptată nevoilor fiecărui pacient.
1. Introduction
Vesicoureteral reflux (VUR) is the retrograde upwards passage of urine from the urinary bladder to the upper components of the urinary collecting system, represented by the ureter, pelvis and calyces. This aberrant flow results in the volume overload of the aforementioned structures, with possible repercussions on the anatomy and function of the reno-urinary system(1,2).
Most frequently, this pathology is diagnosed secondary to a urinary tract infection (UTI) associated with anatomical anomalies of the urinary system detected by ultrasound or after repeated UTIs. Another frequent clinical situation that leads to the diagnosis of vesicoureteral reflux is the postnatal investigation of fetal hydronephrosis detected during pregnancy by ultrasound exam(3,4).
The importance of early diagnosis is given by the need for a correct management to prevent UTIs, the development of renal scars (RS), reflux nephropathy (RN), and the evolution toward chronic kidney disease (CKD)(3,5).
2. Epidemiology
Being the most common urinary tract abnormality, vesicoureteral reflux is estimated to be present in approximately 1-2% of the general pediatric population. One-third of the patients with VUR will experience at least one febrile UTI. On the other hand, up to one-third of children with acute pyelonephritis are later diagnosed with vesicoureteral reflux if adequately investigated(6-8).
Studies have revealed that about 16.2% (between 7% and 35%) of patients with overt antenatal hydronephrosis had vesicoureteral reflux on voiding cystourethrogram (VCUG). Most patients are boys (70%) and generally have a high-grade VUR (about one-third have grade IV-V VUR). They also associate impaired renal function even in the absence of UTIs. Renal structural or functional impairment was found in 6.2% of these patients with low-grade VUR (I-III) and in 47.9% of patients with high-grade VUR (IV-V). On the other hand, patients diagnosed with VUR secondary to UTIs are predominantly female, have lower degrees of vesicoureteral reflux, and the kidney damage is directly proportional to the number of infections. The diagnosis of VUR in the first six months of life is more often associated with males, while children diagnosed after six months are predominantly females. Thus, after the age of 3 years old, the diagnosis of VUR is approximately 10-15 times more frequent in females(2,6,9).
There is also a genetic predisposition to VUR occurrence. Siblings of patients with vesicoureteral reflux have a 25-33% risk of having VUR compared to the general population. In the case of parents with vesicoureteral reflux, the prevalence of VUR in their children can reach 66%, being higher among girls(2,10).
Regarding VUR complications, about 20-30% of patients develop renal scars. The incidence of VUR among patients with end-stage renal disease (ESRD) requiring extrarenal epuration or renal transplantation is approximately 6%, representing the fifth leading cause of ESRD in the pediatric population(2,11,12).
3. Physiopathology of VUR
The normal anatomy of the ureterovesical junction involves the distal ureter entering between the detrusor muscle fibers and traversing a submucosa tunnel before opening into the bladder. For optimal functioning of the junction, the length of this tunnel should be five times the diameter of the distal ureter. If this ratio is lower, the valve mechanism of the junction becomes ineffective, allowing the retrograde flow of urine to occur. This mechanism is responsible for the occurrence of primary vesicoureteral reflux. In the case of secondary VUR, the valve function of the ureterovesical junction is lost due to the increased pressure in the bladder. This phenomenon appears, as mentioned before, in congenital anomalies of the kidney and urinary tract (CAKUT) that cause distal obstruction or in bladder dysfunction that leads to the inability to evacuate urine properly. Moreover, it has been observed that, among boys under 2 years old, bladder pressure is up to four times higher than among older children, leading to the validation of the vesicoureteral reflux, but with a high probability of resolution during growth and development(13-15).
Another mechanism of VUR validation described in the literature is based on the observation that vesicoureteral reflux occurs only within cystitis in certain patients. Local inflammation changes the resistance of the structures involved in the ureterovesical junction. In these cases, VUR remits after the cessation of the inflammatory process(13,14).
The classic pathophysiological theory of the evolution of VUR towards reflux nephropathy assumes the occurrence of high UTIs. Thus, retrograde urinary flow towards the pyelocaliceal system is assumed to facilitate bacterial colonization at this level. The presence of lipopolysaccharides in the structure of uropathogens triggers the immune response via Toll-like receptors (TLRs), which leads to the appearance of local inflammation. In the case of persistent or recurrent inflammation in the renal tissue, the fibrosis process is triggered. Thus, focal fibrotic lesions, known as renal scars, develop in the renal cortex with the evolution toward reflux nephropathy(11,12,14).
However, some patients with VUR develop RN in the absence of UTIs. The physiopathological theory that explains this potential evolution is based on the sterile pressure effect exerted by the refluxed urine on the renal parenchyma. This theory applies to pathologies that cause increased pressure in the bladder and upper urinary collecting system, such as the posterior urethral valve and the neurogenic bladder. It also applies to individuals with high-grade VUR present in the fetal period, in which the sterile pressure effect is exerted on the immature renal structures. In these cases, the renal damage is rather diffuse, with the development of renal cystic dysplasia. Most of the time, these patients are male and represent the majority of cases that evolve with renal function impairment(16-18).
Once developed, renal scars can associate proteinuria and hypertension, potentially evolving towards CKD up to the final stages with the need for extrarenal epuration(11,15).
4. Diagnosis
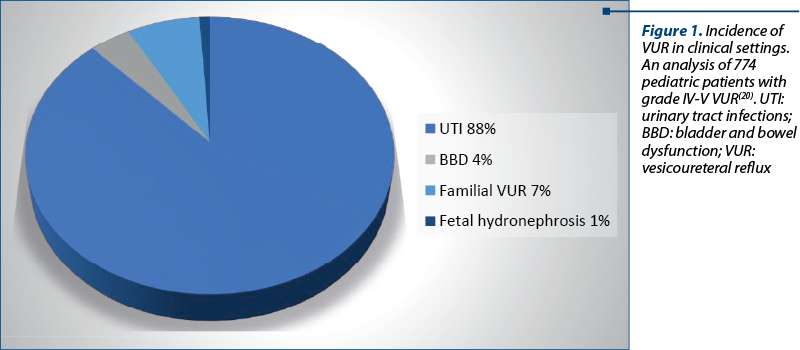
Certain clinical situations suggest the diagnosis of VUR, requiring specific imaging examinations for validation. Thus, vesicoureteral reflux can be diagnosed in patients investigated for fetal hydronephrosis, recurrent UTIs, bladder and bowel dysfunction (BBD), familial VUR, or during the evaluation of CAKUT. Less often, vesicoureteral reflux is detected during the initial evaluation in patients with advanced CKD (Figure 1)(19,20).
The gold standard in diagnosing VUR is VCUG, which, in addition to the presence of retrograde flow of urine at the level of the vesicoureteral junction, brings important information about the anatomy of the urinary tract. Also, it allows the classification of vesicoureteral reflux in five degrees of severity, according to which the therapeutic approach will be guided. This classification is done following the international reflux grading system. Although this examination is an extremely accurate tool in VUR diagnosis, it is an invasive procedure and has the inconvenience of irradiating the patient. Moreover, inevitably repeated exposures will be required in the context of following patients in evolution. In this context, it is desirable to find alternative diagnostic imaging methods. One such alternative is contrast-enhanced voiding ultrasonography (ceVUS). Although this method can reveal the presence of VUR and allows the staging in five degrees of severity, it does not provide reliable information regarding the anatomy of the ureter and the pyelocaliceal system(2,3,21,22).
For the detection of renal scars, the investigation of choice is DMSA renal scintigraphy with Technetium99m. In addition, this investigation also provides information on differential renal function. The patients this investigation addresses are mainly those in whom renal hypoplasia is detected, or cortical scars are suspected on ultrasound(19,23).
It should be mentioned that ultrasonography of the reno-urinary tract is, in most cases, the first imaging investigation performed on these patients. Whether it is a fetal ultrasound or an ultrasound evaluating acute pyelonephritis, this investigation provides quick and valuable information about the anatomy of the urinary tract. Ultrasound changes suggestive of VUR, such as hydronephrosis or ureterohydronephrosis, can be detected. Moreover, the presence of cortical incisions may suggest RS, and if renal hypoplasia is detected, RN and CKD have to be considered. Changes in echogenicity suggest an acute inflammatory process (pyelonephritis) or renal parenchymal fibrosis. In addition, ultrasonography is the most helpful tool for following-up on the evolution of these patients, regardless of the chosen therapeutic approach. It is essential to mention that a normal ultrasound examination cannot exclude the presence of vesicoureteral reflux. Certain studies have shown that 46-60% of patients with VUR have a normal ultrasound examination. In the diagnosis of VUR, ultrasound examination has a low sensitivity (18-46%), a specificity of 76-88%, a negative predictive value (NPV) of 71-83%, and a positive predictive value (PPV) of 24-66%. Thus, even though the ultrasound is normal in a patient with recurrent pyelonephritis and a high suspicion of vesicoureteral reflux, a VCUG is mandatory(19,24-26).
5. Classification
According to the physiopathological mechanism described in detail before, there are two types of vesicoureteral reflux. Primary VUR refers to the solitary dysfunction of the ureterovesical junction that cannot prevent the return of urinary flow upstream. Secondary VUR occurs due to a urinary tract abnormality located downstream of the vesicoureteral junction, which produces an increase in bladder pressure or an increase in urine volume beyond the containment capabilities of the junction. Among the abnormalities that determine secondary VUR, we mention the posterior urethral valve, neurogenic bladder, urethral hypoplasia, and ureterocele(13,27).
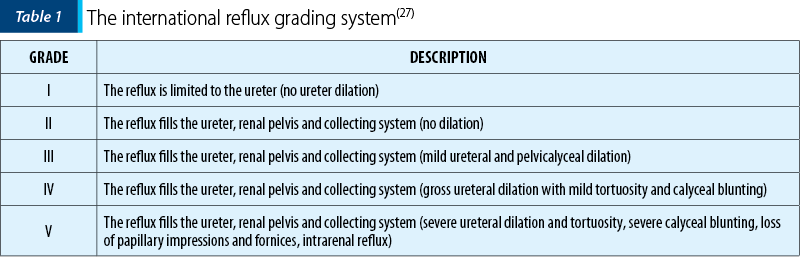
Another classification of vesicoureteral reflux, with implications in the therapeutic approach, is proposed by its appearance in imaging investigations. Thus, according to an international staging system, VUR can be staged in five degrees of severity, depending on the extent of the reflux (Table 1)(27).
6. Management
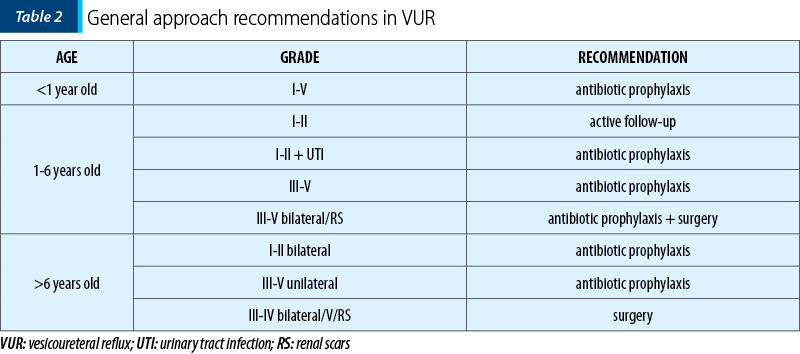
The approach to patients with VUR is complex and should be adapted to each patient, depending on the morphofunctional characteristics and the clinical manifestations. The main objective is the prevention of infectious recurrences, avoiding the evolution towards reflux nephropathy and the occurrence of its complications. On the other hand, a significant percentage of patients with young ages and low degrees of VUR progress to spontaneous remission. Thus, there are three management directions for patients with VUR: active follow-up, antibiotic prophylaxis, and surgical treatment. There is currently no consensus on the optimal approach for these patients, the clinician being the one who must evaluate each case individually to try to offer each patient the treatment that suits his needs (Table 2)(3,19,28,29).
Active follow-up may apply to a limited number of patients. Thus, in young patients, with low-grade VUR (I-II), and in the absence of urinary infections, given the high rate of spontaneous remission (80%), any therapeutic intervention can be delayed. However, these patients will be followed-up by ultrasound, and after 12-18 months, the VCUG will be repeated until the VUR disappears. These patients need to try to avoid the predisposing factors for UTIs. Thus, they must avoid constipation, have an optimal water intake, give up diapers, and follow a micturition program to avoid overloading the urinary bladder that could validate VUR(28,29).
The timing of initiation of antibiotic prophylaxis, as well as its effectiveness in preventing febrile infections and renal scarring, is a subject of ongoing debate. The Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial aimed to evaluate the role of antibiotic prophylaxis in preventing acute pyelonephritis and the development of new renal scars in a group of 607 children with vesicoureteral reflux. The results showed that prophylaxis with trimethoprim-sulfamethoxazole reduced the risk of infectious recurrence by 50%, but without influencing the appearance of new renal scars. The general recommendations are to initiate antibiotic prophylaxis in all patients with high-grade VUR and in those with low-grade VUR associated with UTIs. The recommended dose of antibiotic to use is between a quarter and a third of the curative dose, administered in the evening as a single dose. The treatment will be stopped after proof of remission or after surgical resolution. Although antibiotic prophylaxis is very well tolerated, with no side effects, the selection of multidrug-resistant bacterial strains has been observed in patients who continue to present febrile UTIs(19,28,30-33).
As with antibiotic prophylaxis, the indications for surgical approaches in patients with VUR remain debated. The general indications for choosing a surgical method are the low probability of progression to remission, the increased risk of renal scarring despite antibiotic prophylaxis, and infectious recurrences despite the administration of antibiotic prophylaxis(29,34,35).
For cases with low-grade VUR or even with grade IV, in well-selected cases, endoscopic treatment with injection of antireflux substances is indicated. This procedure is minimally invasive, inexpensive, highly effective, and has a short recovery time. After a first injection, VUR was absent in 79% of cases with grade II, in 72% with grade III, and in 63% with grade IV. The procedure can be repeated, which leads to an increase in efficiency up to 85%. After performing the injection, it is necessary to repeat the cystography every 3-4 months; until that moment, the patient needs to continue antibiotic prophylaxis. In 25% of cases with therapeutic success, vesicoureteral reflux may recur after approximately 12 months, probably due to the resorption or migration of some of the injected material(19,36).
The classic surgical approach involves reimplanting the ureters at the level of the urinary bladder by building a tunnel at the level of the bladder submucosa for the distal ureter, which should be five times longer than the diameter of the ureter. Different surgical techniques are used, with similar results, using either an intravesical or extravesical approach. Regardless of the degree of VUR, the success of classic surgical interventions is about 95-99%. These interventions are recommended for cases with high-grade VUR (IV or V), especially in the presence of renal scars, and in cases with an unfavorable evolution under conservative treatment. The latter are the cases in which infectious recurrences cannot be controlled, there are no signs of improvement in VUR, or the evolution is towards renal scars. A new alternative, which is gaining more and more ground in recent years, is robotically assisted laparoscopic intervention, with results similar to those of classical surgery in terms of success rate(19,34,37).
7. Complications
As mentioned before, the changes produced by vesicoureteral reflux on the renal parenchyma take the form of cortical scars, thus causing the development of reflux nephropathy, characterized by small and irregularly contracted kidneys. Histological findings in RN are global glomerular hypertrophy with periglomerular fibrosis, chronic inflammation with interstitial fibrosis, patchy interstitial scarring, tubular atrophy, and secondary focal segmental glomerulosclerosis (FSGS). In the case of evolution with extensive lesions, nephron mass is lost, which causes hyperfiltration in the remaining nephrons. Thus, the evolution is towards the gradual loss of renal function. The most common complications of reflux nephropathy are proteinuria and hypertension. In addition, hyposthenuria, metabolic acidosis, and progressive CKD may occur(11,15,17,18,38).
Due to histopathological changes secondary to reflux, the renin-angiotensin-aldosterone (RAA) system is activated, which is the mechanism of hypertension in reflux nephropathy. Up to 30% of pediatric patients with renal scars develop hypertension. It is estimated that up to 50% of them will develop hypertension by young adulthood(11,19).
Microalbuminuria has been reported in 51% of children with reflux nephropathy, being a prognostic marker for proteinuria and progressive CKD. Frank proteinuria has been reported in 21% of adult patients with RN, being very rare in children. In addition to these glomerular protein losses, tubular proteinuria occurs in reflux nephropathy, highlighted by the presence of ß2-microglobulin, retinol transporter protein, a1-microglobulin and N-acetyl-ß-D-glucosamine in the urine(11,12).
Reflux nephropathy can have an evolutionary character, with gradual impairment of the renal function and the progression towards CKD until the end stage, with the need for extrarenal epuration. About 10% (between 7% and 17%) of children and young adults with ESRD have VUR as the primary cause. Other studies estimate that up to 25% of cases of CKD among children are due to vesicoureteral reflux, one of the leading causes(2,5,11).
8. Conclusions
Due to its high prevalence in the general pediatric population, vesicoureteral reflux represents one of the most important topics in pediatric nephrology. For the desired therapeutic outcome, early diagnosis is mandatory. The correct selection of patients who require additional imaging investigations is the first step toward a proper management. The main objectives of therapeutic management are to avoid UTIs and the development of renal scars. Thus, the therapeutic approach can be various and complex. There is currently no consensus on the optimal approach for these patients. To increase therapeutic efficacy, the clinical approach to patients with VUR must be individualized based on the morphofunctional characteristics, clinical manifestations, and social and psychoemotional profile. Perhaps, a better understanding of the physiopathological mechanisms – and, thus, improving the therapeutic results – could be obtained through additional studies regarding the genetic and immunological aspects of vesicoureteral reflux.

Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY
Bibliografie
- Williams G, Fletcher JT, Alexander SI, Craig JC. Vesicoureteral reflux. J Am Soc Nephrol. 2008;19(5):847-862. doi:10.1681/ASN.2007020245.
- Miyakita H, Hayashi Y, Mitsui T, et al. Guidelines for the medical management of pediatric vesicoureteral reflux. Int J Urol. 2020;27(6):480-490. doi:10.1111/iju.14223.
- Cooper CS. Diagnosis and management of vesicoureteral reflux in children. Nat Rev Urol. 2009;6(9):481-489. doi:10.1038/nrurol.2009.150.
- Greenbaum LA, Mesrobian HG. Vesicoureteral reflux. Pediatr Clin North Am. 2006;53(3):413-vi. doi:10.1016/j.pcl.2006.02.010.
- Swerkersson S, Jodal U, Sixt R, Stokland E, Hansson S. Relationship among vesicoureteral reflux, urinary tract infection and renal damage in children. J Urol. 2007;178(2):647-651. doi:10.1016/j.juro.2007.04.004.
- Caragata R, Bălănescu L, Bălănescu R, Jinga V. The Epidemiology of Vesicoureteral Reflux in Children - A Nine-Year Retrospective Study. Journal of Urology. 2019;18(1):17-20.
- Venhola M, Hannula A, Huttunen NP, Renko M, Pokka T, Uhari M. Occurrence of vesicoureteral reflux in children. Acta Paediatr. 2010;99(12):1875-1878. doi:10.1111/j.1651-2227.2010.01909.x
- Tekgül S, Riedmiller H, Hoebeke P, et al. EAU guidelines on vesicoureteral reflux in children. Eur Urol. 2012;62(3):534-542. doi:10.1016/j.eururo.2012.05.059.
- Garin EH. Primary vesicoureteral reflux; what have we learnt from the recently published randomized, controlled trials?. Pediatr Nephrol. 2019;34(9):1513-1519. doi:10.1007/s00467-018-4045-9.
- Hewitt I, Montini G. Vesicoureteral reflux is it important to find?. Pediatr Nephrol. 2021;36(4):1011-1017. doi:10.1007/s00467-020-04573-9.
- Mattoo TK. Vesicoureteral reflux and reflux nephropathy. Adv Chronic Kidney Dis. 2011;18(5):348-354. doi:10.1053/j.ackd.2011.07.006.
- Mattoo TK, Mohammad D. Primary Vesicoureteral Reflux and Renal Scarring. Pediatr Clin North Am. 2022;69(6):1115-1129. doi:10.1016/j.pcl.2022.07.007.
- Arena S, Iacona R, Impellizzeri P, et al. Physiopathology of vesico-ureteral reflux. Ital J Pediatr. 2016;42(1):103. Published 2016 Nov 29. doi:10.1186/s13052-016-0316-x.
- Cvitkovic Roic A, Turudic D, Milosevic D, Roic G. Intrarenal reflux with low-grade vesicoureteral reflux: an underestimated significance?. J Ultrasound. 2023;10.1007/s40477-022-00772-2. doi:10.1007/s40477-022-00772-2.
- Andrioli V, Regacini R, Aguiar W. Primary Vesicoureteral reflux and chronic kidney disease in pediatric population. What we have learnt?. Int Braz J Urol. 2020;46(2):262-268. doi:10.1590/S1677-5538.IBJU.2020.02.02.
- Fillion ML, Watt CL, Gupta IR. Vesicoureteric reflux and reflux nephropathy: from mouse models to childhood disease. Pediatr Nephrol. 2014;29(4):757-766. doi:10.1007/s00467-014-2761-3.
- Peters C, Rushton HG. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol. 2010;184(1):265-273. doi:10.1016/j.juro.2010.03.076.
- Cendron M. Reflux nephropathy [published correction appears in J Pediatr Urol. 2009 Feb;5(1):75]. J Pediatr Urol. 2008;4(6):414-421. doi:10.1016/j.jpurol.2008.04.009.
- Miyakita H, Hayashi Y, Mitsui T, et al. Guidelines for the medical management of pediatric vesicoureteral reflux. Int J Urol. 2020;27(6):480-490. doi:10.1111/iju.14223.
- Hunziker M, Colhoun E, Puri P. Prevalence and predictors of renal functional abnormalities of high grade vesicoureteral reflux. J Urol. 2013;190(4 Suppl):1490-1494. doi:10.1016/j.juro.2013.01.068.
- Chua ME, Kim JK, Mendoza JS, et al. The evaluation of vesicoureteral reflux among children using contrast-enhanced ultrasound: a literature review.
- J Pediatr Urol. 2019;15(1):12-17. doi:10.1016/j.jpurol.2018.11.006.
- Kim D, Choi YH, Choi G, et al. Contrast-enhanced voiding urosonography for the diagnosis of vesicoureteral reflux and intrarenal reflux: a comparison of diagnostic performance with fluoroscopic voiding cystourethrography. Ultrasonography. 2021;40(4):530-537. doi:10.14366/usg.20157.
- Balestracci A, Montecuco M, Serviddio C, et al. Role of Late DMSA Renal Scan in Detecting High-Grade Vesicoureteral Reflux. Indian J Pediatr. 2019;86(9):784-789. doi:10.1007/s12098-019-02917-4.
- Kim J, Lim YJ, Yi J, Hahn S, Lee HJ, Shin M, et al. Diagnostic Accuracy of Renal Ultrasonography for Vesicoureteral Reflux in Infants and Children Aged Under 24 Months with Urinary Tract Infections. Journal of the Korean Society of Radiology. 2019;80(6):1179–89. https://synapse.koreamed.org/articles/1138857.
- Naseri M, Karimi M, Bakhtiari E, Tafazoli N, Alamdaran SA, Tafazoli N. Diagnostic Values of Kidney Ultrasonography for Vesicoureteral Reflux (VUR) and High Grade VUR. Iran J Kidney Dis. 2021;15(5):328-335.
- Reed JN, Nicoara O, McMahon BA. Reflux and Obstructive Nephropathy. In: Tubulointerstitial Nephritis [Internet]. 2022 [cited 2023 Apr 17];199–205. Available from: https://link.springer.com/chapter/10.1007/978-3-030-93438-5_15.
- Decter RM. Vesicoureteral Reflux. Pediatr Rev. 2001;22(6):205–10. https://doi.org/10.1542/pir.22-6-205.
- Hajiyev P, Burgu B. Contemporary Management of Vesicoureteral Reflux. Eur Urol Focus. 2017;3(2-3):181-188. doi:10.1016/j.euf.2017.08.012.
- Peters CA, Skoog SJ, Arant BS Jr, et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol. 2010;184(3):1134-1144. doi:10.1016/j.juro.2010.05.065.
- Läckgren G, Cooper CS, Neveus T, Kirsch AJ. Management of Vesicoureteral Reflux: What Have We Learned Over the Last 20 Years?. Front Pediatr. 2021;9:650326. doi:10.3389/fped.2021.650326.
- Bertsimas D, Li M, Estrada C, Nelson C, Scott Wang HH. Selecting Children with Vesicoureteral Reflux Who are Most Likely to Benefit from Antibiotic Prophylaxis: Application of Machine Learning to RIVUR. J Urol. 2021;205(4):1170-1179. doi:10.1097/JU.0000000000001445.
- Thergaonkar RW, Hari P. Current Management of Urinary Tract Infection and Vesicoureteral Reflux. Indian J Pediatr. 2020;87(8):625-632. doi:10.1007/s12098-019-03099-9.
- Blais AS, Bolduc S, Moore K. Vesicoureteral reflux: From prophylaxis to surgery. Can Urol Assoc J. 2017;11(1-2Suppl1):S13-S18. doi:10.5489/cuaj.4342.
- Coco C, Jacobs M. Surgical indications for operative management of vesicoureteral reflux in children. Curr Opin Pediatr. 2021;33(2):243-246. doi:10.1097/MOP.0000000000001000.
- Sung J, Skoog S. Surgical management of vesicoureteral reflux in children. Pediatr Nephrol. 2012;27(4):551-561. doi:10.1007/s00467-011-1933-7.
- Escolino M, Kalfa N, Castagnetti M, et al. Endoscopic injection of bulking agents in pediatric vesicoureteral reflux: a narrative review of the literature. Pediatr Surg Int. 2023;39(1):133. doi:10.1007/s00383-023-05426-w.
- Heidenreich A, Ozgur E, Becker T, Haupt G. Surgical management of vesicoureteral reflux in pediatric patients. World J Urol. 2004;22(2):96-106. doi:10.1007/s00345-004-0408-x.
- Peters C, Rushton HG. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol. 2010;184(1):265-273. doi:10.1016/j.juro.2010.03.076.