Duchenne muscular dystrophy is an X-linked genetic disorder that involves the gene responsible for the synthesis of dystrophin. Decreased dystrophin levels in the body cause a progressive loss in muscle strength that affects skeletal muscle structures and evolves towards disability. Death occurs after a variable period, depending on the case management. Impaired lung function is gradual and progresses to chronic respiratory failure. The severity of respiratory impairment is assessed by careful and periodic monitoring. Along with the information provided by spirometry and muscle inspiratory and expiratory pressures, the application of the pediatric sleep questionnaire, the study of polysomnographic sleep and the monitoring of CO2 levels during sleep are practiced. All these provide information about the impact of this disease on the respiratory system, about the time of sleep respiratory disorders onset and select patients who need support through assisted cough and noninvasive ventilation. The authors present the results of respiratory monitoring in children diagnosed with Duchenne muscular dystrophy and sleep respiratory disorders and observe the impact over time of the noninvasive nocturnal ventilation treatment.
Evaluarea managementului respirator la copiii cu distrofie musculară Duchenne
A review of respiratory management in children with Duchenne muscular dystrophy
First published: 16 martie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.61.1.2021.4714
Abstract
Rezumat
Distrofia musculară Duchenne este o boală genetică cu transmitere X-linkată, care afectează gena implicată în sinteza distrofinei. Nivelurile scăzute ale distrofinei în organism determină scăderea progresivă a forţei musculare, cu afectarea în special a musculaturii striate, şi evoluează către dizabilităţi. Decesul apare după o perioadă variabilă, dependentă de managementul de caz. Afectarea funcţiei respiratorii la pacientul cu distrofie musculară Duchenne este graduală şi evoluează progresiv către insuficienţă respiratorie cronică. Evaluarea dinamicii respiratorii se face prin monitorizare atentă şi periodică. Alături de informaţiile oferite de spirometrie şi de presiunile inspiratorii şi expiratorii musculare, investigaţiile includ aplicarea chestionarului de somn pediatric, studiul de somn polisomnografic şi monitorizarea nivelului de CO2 în timpul somnului. Toate acestea oferă informaţii despre impactul bolii asupra aparatului respirator, despre momentul instalării tulburărilor respiratorii în timpul somnului şi selectează pacienţii care au nevoie de suport prin tuse asistată şi ventilaţie neinvazivă. Autorii prezintă rezultatele monitorizării funcţiei respiratorii la un lot de pacienţi pediatrici diagnosticaţi cu distrofie musculară Duchenne şi tulburări respiratorii în timpul somnului, care au apărut în evoluţia bolii. În plus, este evaluat impactul în timp pe care îl are instituirea tratamentului prin ventilaţie neinvazivă în timpul somnului asupra parametrilor funcţionali respiratori.
Introduction
Duchenne muscular dystrophy (DMD) is a congenital disease characterized by progressive muscle weakness and progressive loss of ambulation. The disease is associated with the gradual loss of respiratory muscle strength, the evolution being invariably lethal due to respiratory failure(1). DMD is caused by mutations in the dystrophin gene that encode dystrophin, a cytoskeletal protein important for the physiological growth of muscle tissue(2). It is a recessive X-linked pathology that occurs almost exclusively in boys. Females are healthy carriers(3). The incidence of the disease is 1:3500 male newborns(3).
The diagnosis is established anamnestically followed by clinical evaluation and associated with increased serum levels of muscle enzymes. It will be confirmed by genetic testing and muscle tissue biopsy.
The patient with DMD must be multidisciplinary managed, considering the multiple organ failure: respiratory, cardiac, digestive, neurological, psychoemotional, endocrinological, ophthalmological.
The respiratory failure that appears in the evolution of the disease is the main cause of mortality and morbidity in patients diagnosed with DMD(4).
The authors believe that the progress in the field of investigating and improving respiratory function in patients with DMD has changed the evolution and quality of life in these patients over the past decade. The last decade has brought a change in the respiratory approach of the patient with DMD by replacing the traditional, noninterventional forms of approach with techniques of active involvement, monitoring and support of these patients.
The most common respiratory pathologies in a patient with DMD are nocturnal hypoventilation and sleep respiratory disorders (respiratory effort-related arousal, apnea, hypopnea). These pathologies appear with a prevalence of over 40% in this group of patients, 10 times higher than in the general pediatric population(4).
The results obtained in our hospital in the management of children with DMD and chronic respiratory failure sustain the importance of supportive respiratory management options.
Materials and method
Sleep evaluation in DMD will determine the onset of respiratory failure, which is always subtle for these patients. The early signs of nocturnal hypoventilation may include fragmented sleep, frequent awakenings, daytime sleepiness, morning headache, behavioral disorders, symptoms that are progressively worsening during the evolution of the disease(1,5). These changes can be diagnosed anamnestically and are supported by paraclinical investigations. These are represented by spirometry, the evaluation of inspiratory and expiratory muscle pressures, together with applying the pediatric sleep questionnaire at each medical visit. The findings must be correlated with the overnight sleep study results (polygraphy, polysomnography) and with the monitoring of CO2 blood levels(1,6).
The sleep questionnaire and the sleep study provide dynamic information related to the failure of respiratory function and allow us to decide the correct timing for initiating nocturnal noninvasive ventilation(7).
The sleep questionnaire includes 22 questions with items in the field of respiratory sleep disorders, sleepiness and behavioral changes. A score higher than 0.33 indicates an increased risk for the presence of sleep respiratory disorders(8). These patients will undergo a sleep study (Figure 1).

Polysomnography is the gold standard for pediatric sleep evaluation, according to the American Association of Sleep Medicine. Polysomnography monitors both physiological parameters and pathological events during sleep(9,10).
Changes noticed in the sleep pathway associated or not with the increase in CO2 levels confirmed by overnight capnography or by blood determination of CO2 levels at awaking time will promote the patient as a candidate for noninvasive ventilation(9).
Results and discussion
The article presents the dynamics of results in the field of respiratory function monitoring due to the progression of neuromuscular disease. We followed-up a group of 25 patients diagnosed with DMD who were under multidisciplinary monitoring at the “Dr. Victor Gomoiu” Clinical Hospital for Children in the period February 2019 – April 2020. An important mention is that these patients were genetically tested to confirm the DMD disease.
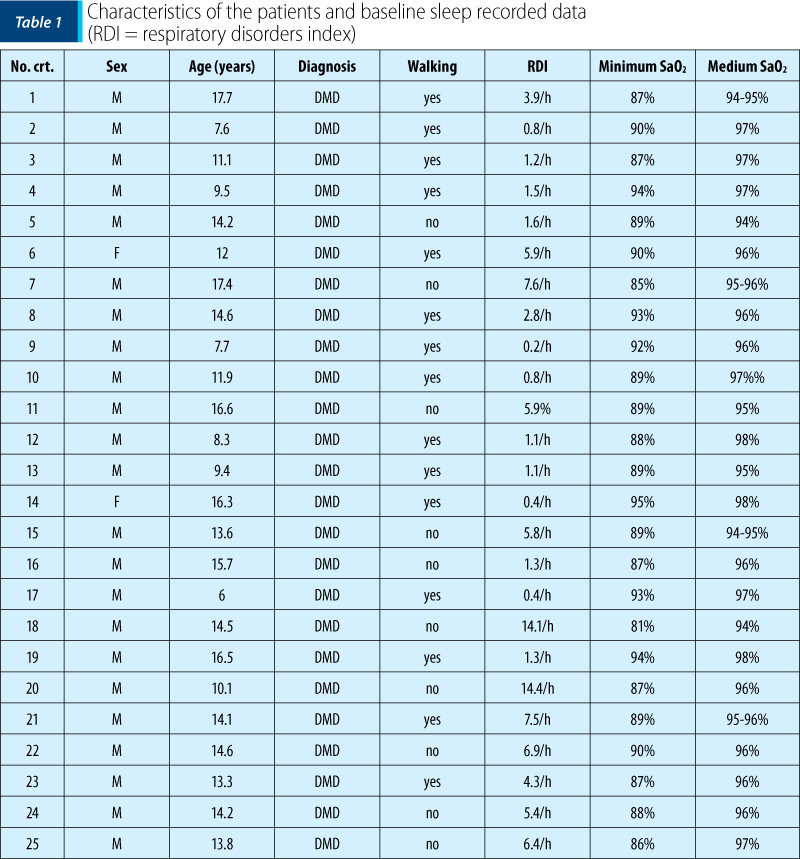
All patients older than 5 years of age and diagnosed with DMD were included in the study. They were reffered to the sleep study either because of the signs and symptoms of sleep-related respiratory problems or due to the underlying disease that is associated with an increased risk to develop sleep-disordered breathing. Table 1 shows the characteristics of the DMD patients and shows baseline of sleep data.
The patients were evaluated by applying the sleep questionnaire, they underwent the polysomnography study, and there were determined the CO2 levels (capnography/blood sample at awakening time) – Figure 2.
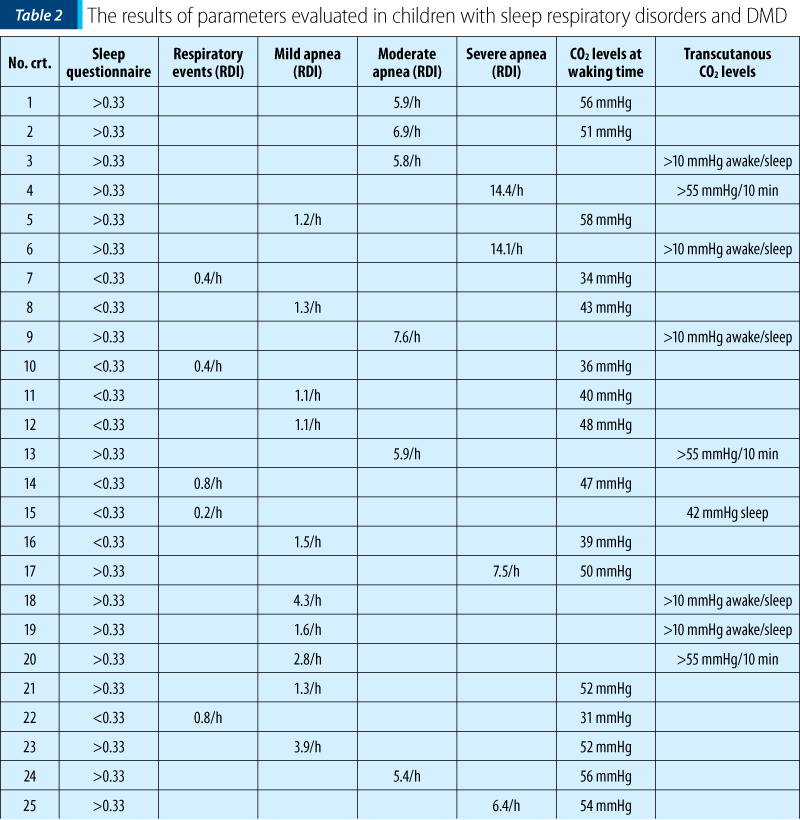
Polysomnography is a sleep study able to highlight the respiratory sleep disorders that occur in DMD patients and allows to stage the severity of events that appear during sleep(5,9). The degree of events severity depends on the number of respiratory events (apnea/hypopnea episodes, upper airway events) that occur per hour of sleep. A mild form of sleep respiratory disorders means respiratory disturbance index (RDI) = 1-5/h, a moderate form means an RDI=5-10/h, and a severe form means an RDI>10/h. These findings are correlated with the other polysomnographically recorded parameters (oxygen saturation level, episodes of tachycardia associated with respiratory events, arousals and awakenings etc.)(5).
Nocturnal hypoventilation is objectified by measuring the level of CO2, in the case of our patients, by Astrup determination on awaking time or by nocturnal transcutaneous measurement of CO2. The American Academy of Sleep Medicine scoring criteria for children hypoventilation suggest:
-
Levels of PCO2>55 mmHg for at least 10 minutes during sleep or
-
Increases of the PCO2 levels ≥10 mmHg as compared to awake supine value up to a level of >50 mmHg for ≥10 minutes or
-
Increases of the PCO2 levels >50 mmHg for >25% of total sleep time(5,12,13).
Also, hypercapnia means levels of PCO2 at awaking time ≥50 mmHg.
Hypoventilation is usually associated with long-term oxygen desaturation, therefore a decrease of SaO2<90% for at least 5 minutes with a nadir of ≤85% may also be an indicator of nocturnal hypercapnia(5,12).
Scoring overnight polysomnography respects the scoring rules of the American Academy of Sleep Medicine. In Table 2, the authors present the results of respiratory parameters in children with sleep respiratory disorders and DMD.
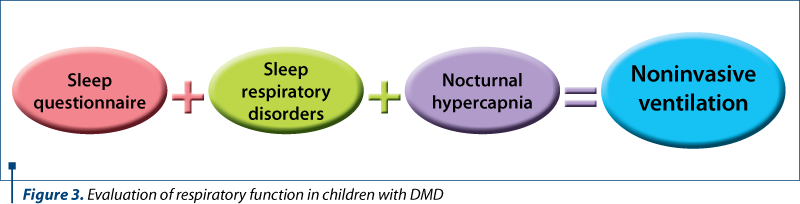
The patients with a positive result at the pediatric sleep questionnaire (score above 0.33), with different degrees of sleep respiratory disorders confirmed by polysomnography and who associate nocturnal hypoventilation confirmed by elevated CO2 values (capnography and blood sample at awakening time), were proposed for noninvasive ventilation. Ventilation parameters were established by titration under polysomnographic guidance (Figure 3).
We followed-up all the patients diagnosed with DMD which were included in the study. Sixteen patients presented changes suggestive for chronic nocturnal hypercapnia, representing 64% of the evaluated group (Figure 4).
This subgroup of 16 patients diagnosed with DMD who associated respiratory impairment confirmed by the sleep questionnaire, characteristic polysomnographic changes and hypercapnia were monitored in dynamic to determine the safety and efficacy of the noninvasive ventilation.
The patients included in the monitored group for noninvasive ventilation efficiency were initiated between February 2019 and April 2020 and then they were monitored clinically and paraclinically, including polysomnography, at intervals established by working protocols: 1 month, 3 months, 6 months. There were slight discontinuities in the evaluation of the patients, caused by the COVID-19 pandemic.
The data provided by the prospective monitoring of patients highlighted a limitation of the progressive chronic degradation of the lung function. Subjectively, the patients with high noninvasive ventilation compliance reported an improvement in the quality of life that cannot be sustained by clinical examination and was inconsistently proven by functional respiratory tests (spirometry, muscle inspiratory and expiratory pressure) performed at each medical visit.
The authors intend to present these results in another paper.
The studies in the literature sustain the importance of evaluation for sleep-related respiratory disorders in patients with DMD due to the impact on morbidity and mortality. Sleep evaluation of respiratory function is used both for screening and therapeutical approach.
In their study, Baskent et al. concluded that children with DMD had a poor sleep quality in the evolution of the disease, because of the pain, physical impairment and respiratory difficulties(13).
The results of the study of Bloetzer et al. highlighted the importance of respiratory evaluation for all children suffering from DMD. They support the idea that respiratory disturbancies during sleep have a high prevalence in DMD patients. Sleep evaluation has a role in determing nocturnal hypoventilation and in detecting the risk factors that can be treated. Polysomnography and monitoring of the CO2 levels are important tools for the assessment of sleep-related respiratory disorders(14).
Conclusions
The sleep department within the “Dr. Victor Gomoiu” Clinical Hospital for Children analyzed 25 patients previously diagnosed with DMD (confirmed by genetic tests) in order to establish the degree of lung damage. The study had both a diagnostic and a therapeutic role. All patients underwent pediatric sleep questionnaire, polysomnography and hypercapnia monitoring.
The results showed:
-
changes in the pediatric sleep questionnaire, with a score >0.33 in 21 patients with Duchenne muscular dystrophy;
-
changes highlighted on the polysomnographic study – varying degrees of apnea, arousals/awakenings, sleep fragmentation, diurnal symptoms (21 patients; 84%);
-
nocturnal hypoventilation (16 patients; 64%).
The patients who simultaneously met hypoventilation criteria with polysomnographic changes and a positive sleep questionnaire were included in the group who received noninvasive ventilation treatment.
The monitoring of the patients included in the group with noninvasive ventilation treatment was done between April 2020 and March 2021, at the intervals supported by the protocol: 1 month, 3 months, 6 months. The evolution of patients who received noninvasive ventilation therapy who were clinically and paraclinically monitored will be published later by the authors.
We are aware that we discuss about a rare genetic disease and that we took into evaluation a limited number of patients. In this pandemic times, it was challenging for us to offer them the right multidisciplinary evaluation. For the future, we want to expand the group of patients with DMD who need respiratory management and to offer them the necessary equipment for monitoring and also for treatment.
The efforts of the multidisciplinary medical team in the management of the patients with DMD can prolong the life of these patients by improving their everyday quality of life.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Finder JD, Birnkrant D, Carl J, Farber HJ, Gozal D, Iannaccone ST, Kovesi T, Kravitz RM, Panitch H, Schramm C, Schroth M, Sharma G, Sievers L, Silvestri JM, Sterni L; American Thoracic Society. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004 Aug 15;170(4):456-65.
- Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep. 2004 Sep;5(9):872-6.
- LaPelusa A, Kentris M. Muscular Dystrophy. 2020 Nov 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID:32809417.
- Katz SL. Assessment of sleep-disordered breathing in pediatric neuromuscular diseases. Pediatrics. 2009 May;123 Suppl 4:S222-5.
- Simonds AK, Backer W, et al. ERS Handbook. Respiratory Sleep Medicine, Page Bros, UK, 2012, pp. 48-52; 120-131; 228-237. DOI: 10.1183/9781849840248-hbsl01
- Sharma GD. Pulmonary function testing in neuromuscular disorders. Pediatrics. 2009 May;123 Suppl 4:S219-21.
- Sharma R, Wolfe L. Use of Non-invasive Ventilation in Neuromuscular Disease. Curr Sleep Medicine Rep. 2017;3:290–298.
- Oros M. Pediatric sleep questionnaire. Available online at: https://www.somn-copii.ro/index.php?option=com_content&view=article&id=38&Itemid=81#default
- Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Ramar K; American Academy of Sleep Medicine. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011 Mar 1;34(3):379-88.
- Chindriş S, Avrămuţă A, Pleşca DA. Obesity and obstructive sleep apnea in children. Proc Rom Acad. 2015; Series B, Supplement 1, 35-40.
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012 Oct 15;8(5):597-619.
- Accardo JA, Shults J, Leonard MB, Traylor J, Marcus CL. Differences in overnight polysomnography scores using the adult and pediatric criteria for respiratory events in adolescents. Sleep. 2010 Oct;33(10):1333-9.
- Baskent G, Demir R, Bektas G, Calskan M. The quality of sleep in Duchenne muscular dystrophy, their mothers and healthy children. ERJ Open Research. 2017 Apr; 3(suppl1):P49.
- Bloetzer C, Jeannet PY, Lynch B, Newman CJ. Sleep disorders in boys with Duchenne muscular dystrophy. Acta Paediatr. 2012 Dec;101(12):1265-9.
Articole din ediţiile anterioare
Caracteristica genotipurilor şi fenotipurilor infecţiei rotavirale în cadrul supravegherii de tip santinelă din Republica Moldova
Potrivit Organizaţiei Mondiale a Sănătăţii, peste 700 de milioane de episoade de diaree la copiii cu vârsta sub 5 ani şi aproximativ 40% din ...
O cauză uitată a durerii cronice abdominale la copii: sindromul de compresiune a nervului cutanat anterior
Chronic abdominal pain (CAP) in adults and children represents a debilitating condition.
Asthma: from complex pathophysiology to histological changes
Astmul este cea mai frecventă afecţiune pulmonară cronică şi afectează aproximativ 15-20% din populaţie în ţările dezvoltate, în timp ce rata est...
Spirometric assessment in asthma in children
Astmul este o boală importantă, atât la adulţi, cât şi la copii, care conduce frecvent la implicaţii financiare şi de sănătate semnificative la...