Acute kidney injury (AKI) is the sudden deterioration of kidney function, which causes a decrease in the glomerular filtration rate. Neonatal acute kidney injury is common in neonates admitted to neonatal intensive care units (NICU) and is a major factor for neonatal mortality and morbidity. The incidence of AKI is 6-24% in NICU neonates, 25% in oliguric forms, 60% in non-oliguric forms and 15% in anuric forms. AKI occurs more frequently in very low birth weight babies. The diagnosis involves family and perinatal history, physical examination of the newborn, biochemical examinations, blood gas analysis, hematological examination, urine measurements, imaging studies and histological studies. Prophylaxis is preferable to curative treatment, and involves early detection of risk factors, fetal ultrasound to detect renal malformations, prevention of decreased renal blood flow or intravascular blood volume by prophylactic administration of dopamine and furosemide or mannitol, along with avoidance of nephrotoxic drugs during pregnancy. Neonatal dialysis in newborns and children who have reduced muscle mass is done at lower values of serum creatinine. Hemodialysis has been replaced by peritoneal dialysis and hemodiafiltration, the technique of choice for the treatment of vascular overload. Peritoneal dialysis is contraindicated in newborns with respiratory distress, shock, peritonitis or ulceronecrotic enterocolitis. Knowing and using standardized definitions and staging of AKI in newborn contribute to a more efficient approach to the patient by the complex medical team.
Leziunea renală acută la nou-născut – o provocare pentru echipa medicală
Acute kidney injury in the newborn – a challenge for the medical team
First published: 31 octombrie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.67.3.2022.7231
Abstract
Rezumat
Leziunea renală acută (LRA) reprezintă deteriorarea bruscă a funcţiei renale, care determină o scădere a ratei de filtrare glomerulară. Leziunea renală acută neonatală este frecventă la nou-născuţii internaţi în unităţile de terapie intensivă neonatală (NICU) şi reprezintă un factor major pentru mortalitatea şi morbiditatea neonatală. Incidenţa LRA este de 6-24% la nou-născuţii din NICU, 25% în formele oligurice, 60% în formele nonoligurice şi de 15% în formele anurice. LRA apare mai frecvent la greutatea foarte mică la naştere. Diagnosticul implică antecedentele familiale şi perinatale, examenul fizic al nou-născutului, examenele biochimice, analiza gazelor din sânge, examenul hematologic, măsurătorile de urină, studii imagistice şi studii histologice. Profilaxia este de preferat tratamentului curativ şi presupune depistarea precoce a factorilor de risc, ecografie fetală pentru depistarea malformaţiilor renale, prevenirea scăderii fluxului sanguin renal sau a volumului sanguin intravascular prin administrarea profilactică de dopamină şi furosemid sau manitol, alături de evitarea administrării de medicamente nefrotoxice în timpul sarcinii. Dializa neonatală la nou-născuţi şi copii care au masa musculară redusă se face la valori mai mici ale creatininei serice. Hemodializa a fost înlocuită cu dializă peritoneală şi hemodiafiltrare, tehnica de elecţie pentru tratamentul supraîncărcării vasculare. Dializa peritoneală este contraindicată la nou-născuţii cu detresă respiratorie, şoc, peritonită sau cu enterocolită ulceronecrotică. Cunoaşterea şi utilizarea definiţiilor standardizate şi a stadializării LRA la nou-născut contribuie la o abordare mai eficientă a pacientului de către echipa medicală complexă.
II. New terminologies
The field of acute renal failure has seen dramatic changes in the last two decades thanks to the increasing recognition of the idea that small changes in kidney function, previously considered of little importance, can have a significant impact both in the short term (length of hospital stay, morbidity), but also in the long term, by the development of chronic kidney disease (CKD). The old, all-or-nothing concept of acute renal failure has been replaced by the term acute kidney injury (AKI). It highlights the progressive nature of an attack on the kidney, the result of which is the failure. The clinician can thus promptly recognize and rapidly intervene in the development of AKI, rather than waiting for the onset of organ failure.
III. Definitions and standardized stages
Acute kidney injury represents the sudden deterioration of kidney function, which causes a decrease in the glomerular filtration rate, the loss of the ability to maintain hydroelectrolyte (HE) and acid-base (AB) balance, and in the ability to eliminate toxic products resulted from metabolism(1). The development of a standardized, multidimensional classification of AKI stages has revolutionized the understanding of the epidemiology and impact of AKI on the long-term prognosis.
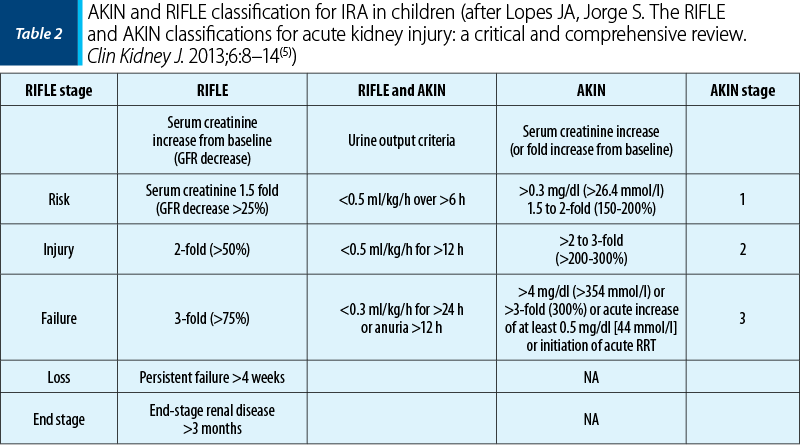
The definition of ARF stages is currently not based on the absolute value of creatinine, but on:
-
changes in serum creatinine level (or estimated creatinine clearance), starting from a previous patient’s baseline;
-
the level of diuresis.
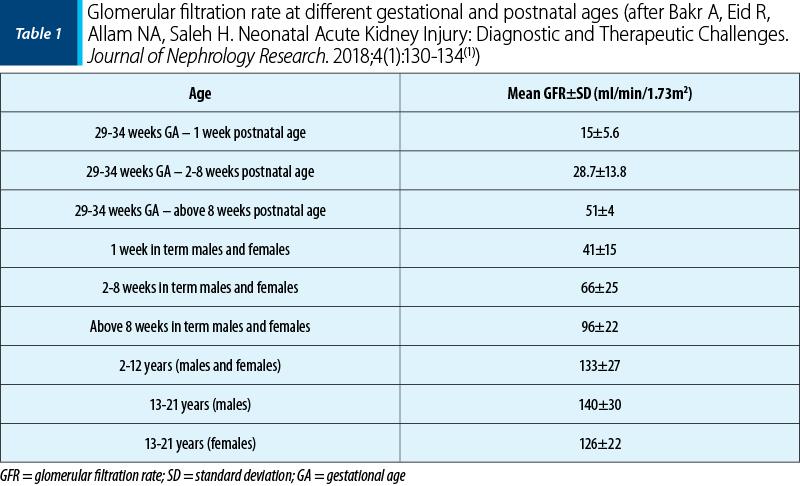
The current staging use the Schwartz formula(4) to define AKI:
Creatinine clearance (ml/min/1.73 sq m) =
k x height (cm)/serum creatinine (mg/dl)
k=0.45 for 0-1 years old; k=0.55 for 1-12 years old;
k=0.55 for 12-18-year-old girls;
k=0.7 for 12-18-year-old boys
Previous baseline creatinine is an important parameter in the staging of AKI, hence the importance of including creatinine in the routine analysis of an evaluation. If this value is not known, it can be calculated in reverse, using the formula:
eGFR normal 120 ml/min =
0.413 x height (cm)/creatinine (mg/dl)
Since the beginning of the 2000’s, pediatric nephrologists have been using the Acute Kidney Injury Network classifications (AKIN classification, 2007) and the “staging system and the risk, injury, failure, loss and end-stage renal disease classification” (RIFLE classification, 2004), which allows an improved diagnosis and staging of AKI according to severity(5).
Later, in 2012 and 2013, Jetton and Askenazi(6) and Ricci and Ronco(7), respectively, proposed a standardized definition of neonatal renal failure based on the Kidney Disease: Improving Global Outcomes (KDIGO) definition for adults and children.
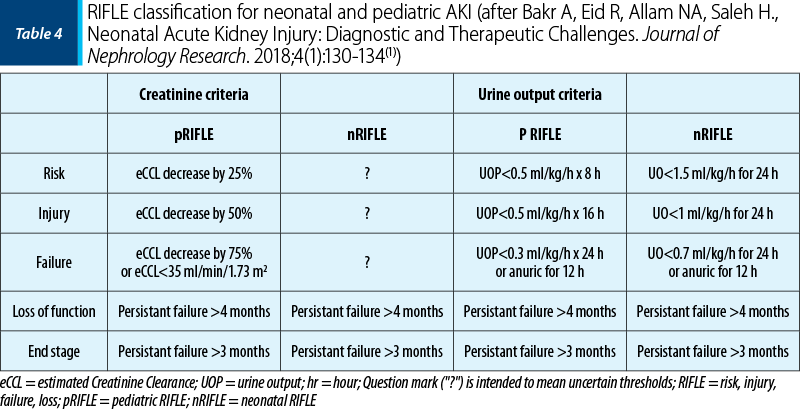
The estimation of baseline serum creatinine is challenging in neonates because serum creatinine declines rapidly in the first week of life as nephron function matures and maternal creatinine is cleared. A change in diuresis is the earliest clinical sign of AKI and it is the simplest method of diagnosing AKI in newborns, therefore oliguria is considered a specific sensitive marker of AKI in newborns. Table 4 compares the pediatric and neonatal RIFLE criteria(1).
IV. Epidemiology and incidence of AKI
in newborns
Neonatal acute kidney injury is common in neonates admitted to neonatal intensive care units (NICU) and is a major factor in neonatal mortality and morbidity(2). The incidence of AKI is 6-24% in newborns in intensive care units, 25% being represented by oliguric forms, 60% being non-oliguric, and 15% anuric forms. Premature newborns (especially those with birth weight below 1500 g) and newborns with sepsis, asphyxia or cardiovascular malformations (persistent ductus arteriosus, transposition of great vessels) are at an increased risk of developing acute renal failure. Acute kidney injury associated with kidney malformations has an incidence of 50%. Genetic factors that increase the risk for AKI are mutations of the gene that encodes the angiotensin enzyme,or its receptor, which cause changes in the activity of the renin-angiotensin-aldosterone system (RAAS), which ultimately leads to AKI(8). Acute kidney injury occurs more frequently in very low birth weight (VLBW) newborns who have mutations in the genes encoding the heat shock protein 72(9). Since this protein has an important role in the production of renal ischemic injury, these findings suggest that some newborns would be more susceptible to ischemic injury.
The mortality reflects trends similar to those of older children and adults. Non-oliguric acute renal failure is associated with a higher survival rate than oliguric acute renal failure. Norman and Asadi observed a mortality rate of 45% in oliguric renal failure, and other authors noted a mortality rate between 14% and 73%(10).
The international, retrospective, observational, cohort study AWAKEN assessed the epidemiology of AKI worldwide in neonates exclusively admitted to neonatal intensive care units and who received at least 48 hours of intravenous volume resuscitation(11). Early AKI was defined by an increase in serum creatinine of 0.3 mg/dl, or a decrease in diuresis below 1 ml/kg per hour on postnatal days 2-7. Risk factors for AKI and the association with length of hospital stay and mortality were assessed. Twenty-one percent (449 of 2110) developed early AKI, which is associated with a higher risk of death. Factors associated with a higher risk of AKI included: preterm birth, resuscitation with epinephrine, marked hyperbilirubinemia, inborn errors of metabolism, or the need for emergency neonatal surgery. Risk factors varied by gestational age. The study showed that 50% of newborns with asphyxia presented prerenal damage, 17% of them presented oliguria and increased creatinine, and 11% presented only increased azotemia. The same authors observed the association of prolonged oliguria with chronic kidney disease, hypoxic-ischemic encephalopathy and with long-term neurological deficits(12).
V. Etiology of AKI in the newborn
The most common form of AKI in neonates is prerenal failure due to renal hypoperfusion or ischemia. Prerenal AKI can lead to intrinsic renal failure if not treated promptly. The newborn kidney is particularly sensitive to hypoperfusion due to the physiological characteristics of the neonatal kidney, including high renal vascular resistance, high plasma renin activity, low glomerular filtration rate, low intracortical perfusion rate, and decreased proximal tubule sodium reabsorption in the first days of life(13). Thus, newborns can develop more easily acute tubular necrosis or cortical necrosis. The etiology of AKI in the newborn is multifactorial. In most studies, birth asphyxia and sepsis are the most commonly associated conditions. Other conditions associated with the development of AKI in the newborn include respiratory distress syndrome, dehydration, congestive heart failure and nephrotoxic drugs(14), but also the obstructive causes, like posterior urethral valve, phimosis, preputial imperforation, urethral diverticulum, blood clot, prune belly syndrome and extrinsic bladder obstructions by tumors, mesenteric cyst, adrenal hemorrhage(15).
A special place in the classification of acute kidney injury in the newborn should be given to hospital-acquired acute renal dysfunction. In developed countries, AKI has been intensively studied in children with operated congenital heart malformations, but also in non-cardiac patients with severe diseases from intensive care units. In them, the incidence of AKI varies between 30% and 60%(16). Thus, the term hospital-acquired AKI (HA-AKI) appeared, which signifies an episode of AKI due to a renal involvement that occurred in the patient after hospitalization, as opposed to community-acquired AKI (CA-AKI), in which the initial event occurred before hospitalization. In developed countries, nosocomial infection and primary renal disease remain the main cause of CA-AKI, instead the etiology of HA-AKI is often multifactorial and reflects comorbidities (for example, bone marrow transplantation, healthcare-associated infections, hospitalization in neonatal intensive care units).
VI. Current role of urinary biomarkers
The early diagnosis of acute renal dysfunction in the newborn may bring new therapeutic perspectives. Knowledge of serum and urinary biomarkers involved in the pathophysiology of newborn AKI may change the approach to this diagnosis, helping to differentiate causes and rapidly implement preventive interventions. Serum creatinine and diuresis are functional biomarkers of AKI, they don’t assess the presence or absence of tissue structural lesions, and only quantify the decrease in the glomerular filtration rate. In addition, their change appears after 72 hours from the onset of renal injury. Animal models have identified proteins secreted in the urine, as a result of cellular damage to the renal tubules, involved in the pathophysiology of AKI and which allow the early identification of tissue damage, before the function is affected. Studies of specific biomarkers in neonatal acute kidney injury are limited and performed mainly in risk populations, such as low birth weight preterm infants, perinatal asphyxiated infants, and those undergoing cardiopulmonary surgery(17). Studies conducted in children who underwent heart surgery or in seriously afflicted patients from intensive care units, who received substances with renal toxicity demonstrated the increase in the urinary concentration of these biomarkers 48 hours before the increase in serum creatinine. Moreover, marked increases predict a greater degree of severity of AKI(18).
VII. Diagnostic considerations
Diagnostic considerations involve family and perinatal history, physical examination of the newborn, biochemical examinations, blood gas analysis, hematological examination, urine measurements, imaging studies and histological studies (where required).
Family history is important when detecting cystic disease or cases of kidney disease associated with hearing impairment. Likewise, the presence in a sibling of perinatally deceased children should draw attention to cases of plurimalformative syndromes, or to congenital nephrotic syndromes, recessive polycystic diseases or renal hypodysplasia. The identification of maternal risk factors such as diabetes is important, the disease being linked to the association of macrosomic baby, with an increased risk of prenatal asphyxia, a favorable condition for the association of acute renal injury through a hypoxic-ischemic mechanism. The association of hypertrophic cardiomyopathy was found more frequent in newborns of diabetic mothers, in the same chronic hypoxic context. The evoked mechanisms are related to the transplacental transport of low oxygen in conditions of increased need due to fetal hyperinsulinism. This can lead to fetal hypoxia and increased fetal erythropoietin, which also explain polycythemia in newborns of diabetic mothers(19). Described since the 1950’s, renal vein thrombosis is a severe perinatal complication that occurs more frequently in newborns of diabetic mothers, related to episodes of hyperosmolar dehydration(20).
The retrospective evaluation of amniotic fluid can be suggestive of a renal obstructive pathology possibly causing AKI. The presence of oligohydramnios may suggest renal agenesis/hypoplasia, or posterior urethral valve, while polyhydramnios may point to the VACTERL association, diabetes insipidus or Bartter syndrome (polyuria, hypokalemia and metabolic acidosis). Antenatal ultrasound is becoming an increasingly used tool in the morphological assessment of the kidney.
Converting enzyme inhibitors or nonsteroidal anti-inflammatory drugs (indomethacin) given to the mother can produce potentially reversible oliguric renal failure. Severe and sometimes irreversible AKI has been described in neonates exposed to indomethacin and, more recently, to nimesulide or non-selective cyclooxygenase (COX) inhibitors, during fetal life(21). For these reasons, in 2020, FDA warned that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) around 20 weeks or later in pregnancy may cause rare but serious kidney problems in an unborn baby(22).
The physical examination of the newborn is important for objectifying changes in the context of Potter or prune belly syndromes, chromosomal diseases (11, 13, 18, 21 trisomy), bladder exstrophy, and sexual ambiguity in the context of 21 hydroxylase deficiencies. Bimanual renal palpation can highlight abdominal masses that can be associated with polycystic kidney, giant hydronephrosis, renal vein thrombosis or, very rarely, congenital nephroblastoma (Wilms tumor).
Biochemical tests are also important in establishing the diagnosis of acute kidney injury. After birth, serum creatinine reflects maternal renal function and cannot be used as an indicator for the newborn. In the full-term newborn, the glomerular filtration rate increases rapidly, and the serum creatinine drops to 0.4-0.6 mg/dl two weeks after birth. This decrease occurs more slowly in premature babies (up to six weeks). The use of serum creatinine as a marker for renal failure must be corroborated with gestational age and postnatal age. Changes in serum creatinine are gross compared to those of renal function, and recent studies seek to define new biomarkers that allow the detection of AKI before changes in serum creatinine. The increase of serum creatinine by more than 0.2 mg% per day and of BUN by more than 10 mg% per day is a classic indicator of acute renal failure.
Dosing electrolytes is essential for diagnosis, but also for monitoring the evolution of renal failure in newborns. The increase in sodium is observed only in prerenal uremia, severe dehydration or inadequate fluid intake. Hyponatremia is usually noted, sometimes severe, with the risk of generating convulsive events. The sodium excretion fraction is normally below 2.5. Values above 2.5-3% suggest parenchymal AKI. Hyperkalemia is especially associated with hypoxic-ischemic injury and obstructive uropathy. Magnesium and phosphorus are not changed initially, but must be monitored. The dosage of uric acid is of interest especially in acute post-asphyxia renal injuries. The examination of blood gases and acid-base balance usually showed an associated metabolic acidosis.
The hematological examination is important for the diagnosis of polycythemia (with thrombosis risk) and disseminated intravascular coagulation, associated with an increased risk of acute renal failure.
Volume overload is an essential clinical-biological parameter to monitor in newborns admitted to neonatal intensive care units. The clinical examination is suggestive most of the time, through weight gain (which must be monitored daily), decreased diuresis, signs of systemic circulatory overload (tachycardia, arterial hypertension), and complications such as acute pulmonary edema or pulmonary hypertension. In a recently published cohort study, Askenazi et al. demonstrated the direct relationship between acute kidney injury, volume overload and neonatal mortality(23). Thus, a volume overload of up to 40% of the initial weight in a newborn with acute renal injury predisposes to a mortality of over 77%, compared to newborns in which the renal dysfunction evolves with euvolemia or hypovolemia, without significant differences depending on gestational age or birth weight. Daily arterial pH and weight monitoring thus become the monitoring parameters of the newborn with acute renal dysfunction.
VIII. Practical therapeutic approach
in the emergency department
Prophylaxis is preferable, of course, to curative treatment and involves early detection of the aforementioned risk factors, fetal ultrasonography to detect renal malformations, prevention of decreased renal blood flow or intravascular blood volume by administering prophylactic dopamine and furosemide or mannitol, and avoiding the nephrotoxic drugs administration during pregnancy. Theophylline infusion, which is indicated in the first hour after birth in severe asphyxia, was associated with restoring the water balance and reducing the serum creatinine level from the third day of life, with no effect on neurological or respiratory complications (Jenik AG, 2016). But newborns receiving theophylline tend to have a high incidence of persistent pulmonary hypertension (Lemley KV, 2016). In the study published in 2019, Bhatt and colleagues established, following the analysis of nine trials, the importance of administering a dose of theophylline for AKI prophylaxis in newborns(24).
The treatment involves general supportive treatment with hydroelectrolytic, acid-base and nutritional balancing, blood pressure control, cardiac output optimization, as well as the treatment of the primary cause (asphyxia, infections, shock etc.).
Neonatal dialysis in newborns and children who have reduced muscle mass – the initiation of renal replacement is done at lower serum creatinine values. Hemodialysis has been replaced by peritoneal dialysis and hemodiafiltration (SCUF), the technique of choice for the treatment of vascular overload. Peritoneal dialysis is contraindicated in newborns with respiratory distress, shock, peritonitis or ulceronecrotic enterocolitis. A recent study showed that the choice of renal replacement method by the pediatric nephrologist included 30% peritoneal dialysis, 20% hemodialysis, and 40% hemofiltration(25). Kidney transplant is not an available option at this age.
Nutrition aims to minimize the excessive catabolism. The minimum caloric intake will be 100 kcal/kg/day, with protein in a dose of 1-2 g/kg/day. If the newborn cannot be enterally fed, with breast milk, total parenteral nutrition is instituted in which the usual amino acid solution must be replaced by essential amino acids supplemented with L-histidine in a dose of 0.5-1 g/kg/day. Dosage adjustment of drugs with renal elimination should be performed such as to avoid toxic levels either by extending the administration interval or by reducing doses. Administration of drugs such as aminoglycosides, acyclovir, amphotericin B, cyclosporins, paralyzing agents, tolazoline, antiepileptic drugs and digoxin can increase the renal damage.
IX. Prognosis
The prognosis depends on the etiology of AKI. The factors associated with increased mortality are multiorgan failure, hypotension, the need to use vasopressors, hemodynamic instability, the need for mechanical ventilation and the need for extrarenal purification by dialysis. The immediate mortality of infants with urinary tract malformations depends on their association with other syndromes (Potter syndrome, severe pulmonary hypoplasia, renal agenesis, prune belly syndrome). The mortality rate in newborns with oliguric renal failure was 60% in cases due to asphyxia and sepsis(10). A higher rate was recorded in those with congenital heart malformations and multiorgan failure. In children with peritoneal dialysis for AKI, the reported mortality is 64% in those with oligoanuria, compared to 20% in those with normal diuresis(25). The long-term follow-up of children with AKI revealed that CKD can install after 3-5 years, suggesting that the effects of AKI are in time, on nephron total mass. Newborns with renal congenital diseases (renal dysplasia, obstructive uropathy, cortical necrosis, polycystic renal disease) are at a high risk to develop chronic kidney disease. In the short term, the prognosis depends on the general condition of the newborn and the condition of all major organs and systems. It is essential to appreciate the newborn as a whole, and not just the renal pathology. The prognosis for non-oliguric renal failure or prerenal failure is better than for those with renal failure of renal origin. The long-term effects are also related to the moment when the offending factor acts. AKI before 34 weeks of gestation leads to a reduction in the total number of nephrons. AKI in prematurity or in small for gestation age children is associated with reduced GFR, proteinuria after a few years, and with the risk of renal hypertension as teenagers.
X. Conclusions
The association of AKI is frequent in newborns from pediatric intensive care units and markedly increases the risk of mortality. Contrary to the classical theory (full recovery is the rule), the patient who went through AKI has a risk of developing CKD and requires long-term follow-up. The long-term prognosis must be addressed in a team that includes a neonatologist, nephrologist, anesthesiologist and intensive therapist, urologist, clinical geneticist and radiologist. Knowing and using standardized definitions and staging of AKI in newborns contribute to a more efficient approach to the patient by the complex medical team.
Author contributions: All authors have contributed equally to this work.
Acknowledgments: The authors want to thank to Lecturer Dr. Bogdan Stana for his suggestions and help for the publication of this paper.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Bakr A, Eid R, Allam NA, Saleh H. Neonatal Acute Kidney Injury: Diagnostic and Therapeutic Challenges. Journal of Nephrology Research. 2018;4(1):130-134.
-
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–48.
-
Moritz KM, Wintour EM. Functional development of the meso- and metanephros. Pediatr Nephrol. 1999 Feb;13(2):171-178.
-
Schwartz G, Brian LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children and adolescent. Pediatrics Cain North Am. 1987;34(3):571-590.
-
Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013 Feb;6(1):8–14.
-
Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24(2):191-196.
-
Ricci Z, Ronco C. Neonatal RIFLE. Nephrol Dial Transplant. 2013;28(9):2211-2214.
-
Summary of Recommendation Statements. Kidney Int Suppl (2011). 2012 Mar;2(1):8-12. doi: 10.1038/kisup.2012.7.
-
Fekete A, Treszl A, Tóth-Heyn P, et al. Association between heat shock protein 72 gene polymorphism and acute renal failure in premature neonates. Pediatr Res. 2003;54(4):452-455.
-
Gorga S, Murphy H, Selewski D. An Update on Neonatal and Pediatric Acute Kidney Injury. Current Pediatrics Reports. 2018;6:278–290.
-
Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184-194.
-
Charlton JL, Boohaker L, Askenazi D, et al. Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol. 2019 Feb 7;14(2):184–195.
-
Basile D, Sreedharan R, Van Why S. Part X: Pathogenesis of Acute Kidney Injury. In: Avner E, Harmon W, Niaudet P. Pediatric Nephrology, seventh edition. 2016, 2099–2138.
-
Youssef D, Abd-Elrahman H, Shehab M, Abd-Elrheem M. Incidence of Acute Kidney Injury in the Neonatal Intensive Care Unit. Saudi J Kidney Dis Transpl. 2015;26(1):67-72.
-
Stârcea M. Insuficienţa renală acută. In: Iordăchescu F, Georgescu A, Miron I, Mărginean O. Tratat de Pediatrie, Editura ALL, 2019, cap.6, 1359-1369.
-
Li S, Krawczeski C, Zappitelli M. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery – a prospective multicenter study. Crit Care Med. 2011 Jun;39(6):1493–1499.
-
Libório AB, Branco KM, Torres de Melo Bezerra C. Acute kidney injury in neonates: from urine output to new biomarkers. Biomed Res Int. 2014;2014:601568.
-
Bihorac A, Guiding AKI. Prevention Using Biomarkers. Crit Connect. 2015;14(2):1–11.
-
Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes?. World J Diabetes. 2015 June 10;6(5):734-743;
-
Winyard PJ, Bharucha T, De Bruyn R, et al. Perinatal renal venous thrombosis: presenting renal length predicts outcome. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F273-F278.
-
Benini D, Fanos V, Cuzzolin L, Tatò L. In utero exposure to nonsteroidal anti-inflammatory drugs: neonatal renal failure. Pediatr Nephrol. 2004;19(2):232-234.
-
https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-recommends-avoiding-use-nsaids-pregnancy-20-weeks-or-later-because-they-can-result-low-amniotic, 2020.
-
Askenazi DJ., Koralkar R., Hundley H, Montesanti A, et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol. 2013 April;28(4):661–666.
-
Bhatt GC, Gogia P, Bitzan M, Das RR. Theophylline and aminophylline for prevention of acute kidney injury in neonates and children: a systematic review. Arch Dis Child. 2019;104(7):670-679.
-
Mian AN, Askenazi DJ, Mhanna MJ. Therapeutic Options for Neonatal Acute Kidney Injury (AKI). Curr Treat Options Peds. 2016;2:69–81.
Articole din ediţiile anterioare
Maternal preeclampsia effects on newborn – review
Preeclampsia este o afecţiune frecventă în prezent, până la 5-10% dintre sarcini fiind asociate cu această boală. Pe lângă efectele devastatoare ...
Icterele neonatale
Icterul apare la nou-născut la o valoare de peste 5 mg/dl bilirubină totală, faţă de adult, la care icterul este vizibil la valori ale bilirubinei ...
Suflul cardiac la copil – când să ne îngrijorăm?
În era ecocardiografiei, suflurile cardiace constituie cea mai frecventă cauză de prezentare la medicul cardiolog pediatru. În aceste condiţii, maj...
Icterul în prima lună de viaţă - un fapt banal sau o provocare medicală
În perioada primei luni de viaţă, icterul reprezintă cel mai comun semn în atenţia medicului care are în grijă nou-născutul. Coloraţia galbenă a te...