Retinopathy of prematurity is a proliferative disorder of the developing retinal blood vessels in premature infants. It is the most common complication of prematurity, especially in extremely preterm infants. The neonatologist’s role in preventing is important, because retinopathy of prematurity is the second leading cause of childhood blindness in the world. The strongest predictors for retinopathy of prematurity are lower gestational age and birth weight. Prolonged administration of oxygen, IUGR, and duration of assisted ventilation are also associated with retinopathy of prematurity.
Retinopatia de prematuritate - actualizare de screening şi management
Retinopathy of prematurity - an update on screening and management
First published: 15 noiembrie 2016
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
a dezvoltării vaselor sangvine retiniene la nou-născuţii prematuri. Este una din cele mai frecvente complicaţii ale prematurităţii, în special la prematurii foarte mici. Rolul neonatologului în depistarea precoce este important, deoarece retinopatia de prematuritate este a doua cauză de orbire la copii în lume. Cei mai puternici predictori ai retinopatiei de prematuritate sunt vârsta gestaţională mică şi greutatea mică la naştere. Administrarea îndelungată de oxigen, întârzierea în creşterea intrauterină, durata ventilaţiei asistate sunt de asemenea asociate cu retinopatia de prematuritate.
Pathophysiology
In the normally developing retina, there are no retinal vessels until about 16 weeks of gestation.
Retinal vascularization begins at the optic nerve at 16 weeks of gestational age (GA) and is completed by 40 weeks GA.
Preterm infants have incompletely vascularized retinas. ROP is a biphasic disease consisting of an initial phase of vessel growth cessation and loss followed by a second phase of vessel proliferation.
- Phase 1 appears from birth to aproximately 30-32 weeks of postmenstrual age (PMA); concentrations of insulin, like growth factor (IGF 1), are low and suppresses vascular endothelial growth factor (VEGF).
- Phase 2 starts at 32-34 weeks PMA. Insult and high oxygen exposure released by the hypoxic retina, VEGH are increase and appears retinal neovascularization.
Risk factors
The most significant risk factor of ROP is extreme prematurity. Birth characteristics and postnatal risk factors may also lead to the development of ROP. Younger GA and low birth weight, white race, and multiple birth increase the risk of severe ROP.
Postnatal risk factors include excessive or fluctuating oxygen levels (oxygen monitoring is an important part of the care of preterm infants), respiratory distress, hypercapnia and hypocapnia, exchange transfusion and anemia, sepsis and intrauterine infections, hyperglicemia, prolonged parenteral nutrition, lactic acidosis, low IGF 1 levels, low omega-3 fatty acids, and low energy delivery.
For every 10 kcal/kg/day increase in energy intake, there was an associated 24% decrease in severe ROP(12). Total energy was indirectly associated with the risk of ROP.
The effect of this nutritional parameters was evident in the first four postnatal weeks(1).
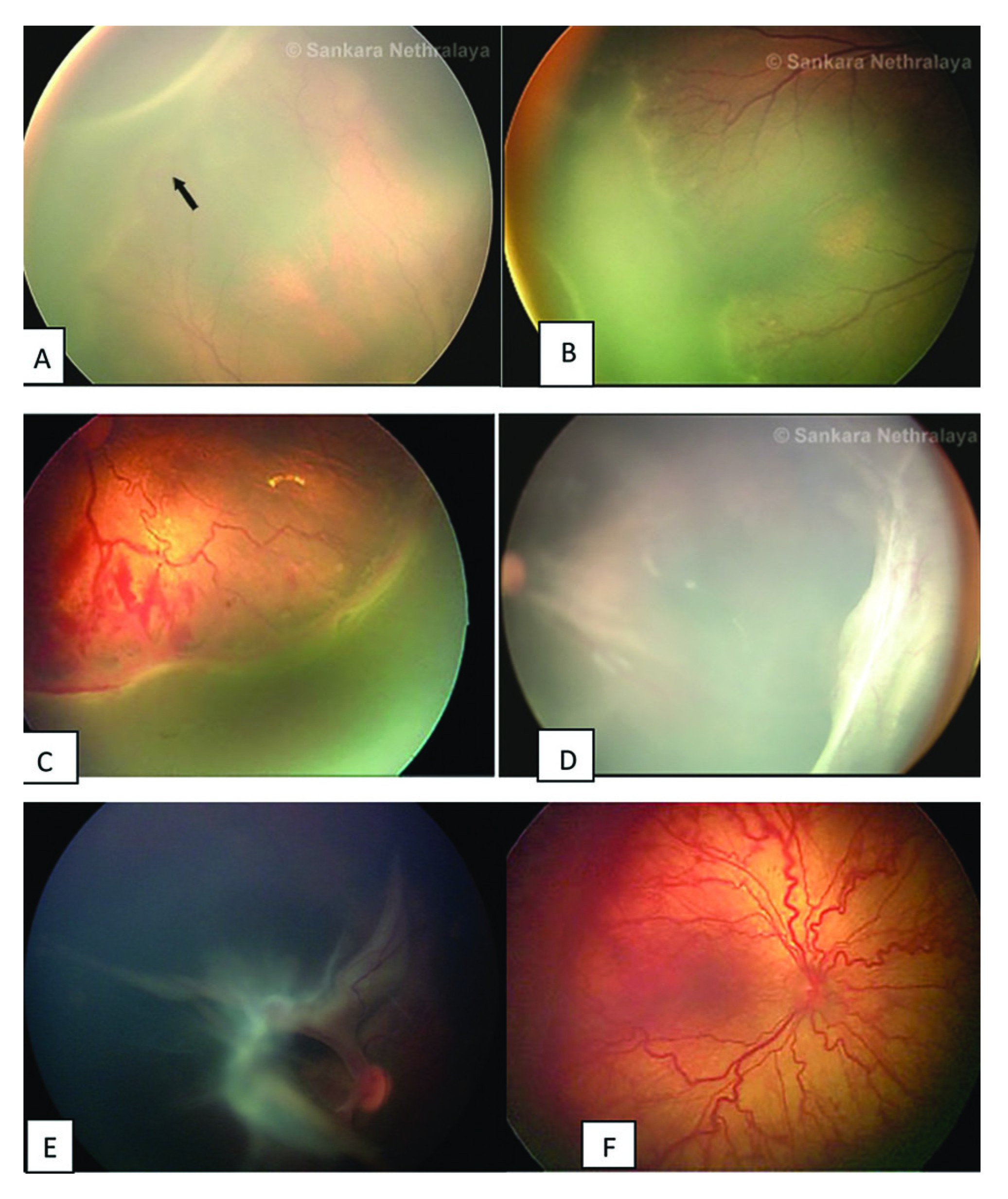
Classification of ROP
ROP stages - there are 5 stages:
- Stage 1: flat demarcation line.
- Stage 2: ridge (demarcation line with height and width).
- Stage 3: ridge with extraretinal neurovascularization that extends into the vitreous.
- Stage 4:
substage 4B - fovea involving retinal detachment.
- Stage 5: complete retinal detachement.
Diagnosis
ROP screening
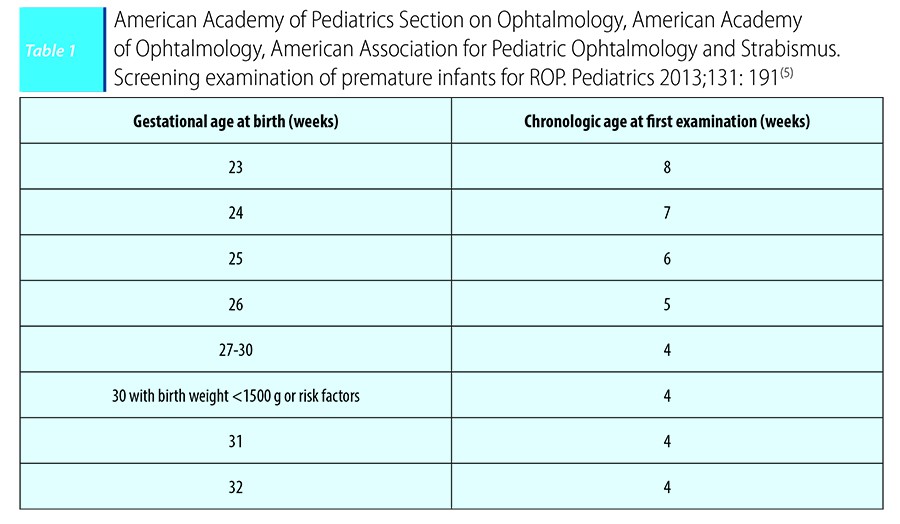
The time of the first examination should be based on menstrual age rather than postnatal age.
1. neonate born at 26 weeks of gestation;
2. neonate born at 29 weeks of gestation with a birth weight of 1000 g;
3. neonate born at 36 weeks of gestation with a birth weight of 1499 g.
4. neonate born with a birth weight <1500 g or GA<30 weeks.
5. neonate born with birth weight between 1500 g and 2000 g or GA>30 weeks who had an unstable clinical course and are belived to be at risk for severe ROP(3,10).
Screening guidelines - Indian scenario(4)
- birth weight <1700 g;
- gestational age at birth <34-35 weeks;
- exposed to oxygen >30 days;
- infants born at <28 weeks and weighting <1200 g are particulary at high risk of developing severe form of ROP;
- other factors: respiratory distress syndrome, sepsis, multiple blood transfusions, multiple births (twins/triplets), intraventricular hemorrhage. In these cases, screening should be considered even for babies >37 weeks of gestation or >1700 g birth weight(9).
The moment for the initial ophtalmologic examination to screen for ROP is based on postmenstrual age for preterm infants of lower gestational age, taking a longer time to develop significant ROP (Table 1).
Guidelines on timing for ROP screening
In infants with specific retinal findings, with significant disease, after this initial screening examinations, it is required a careful follow-up(3).
ROP follow-up
ROP follow-up examinations:
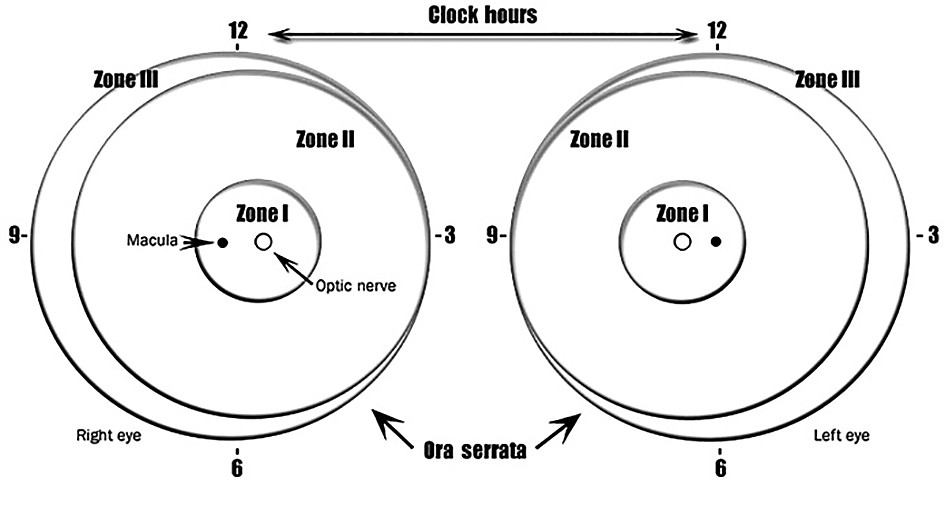
1 week or less follow-up for:
- Zone I or border of zones I and II, immature vascularization.
- Zone I, stage 1 or 2 ROP.
- Zone II, stage 3 ROP.
- Presence or suspected presence of aggressive posterior ROP.
1 to 2 weeks follow-up for:
- Posterior zone II, immature vascularization.
- Zone I, regressing ROP.
- Zone 2, stage 2 ROP.
2 weeks follow-up for:
- Zone II, immature vascularization.
- Zone II, regressing ROP.
- Zone II, stage 1 ROP.
2 to 3 weeks follow-up for:
- Zone III, stage 1 or 2 ROP.
- Zone III, regressing ROP.
ROP screening continues until:
- Full retinal vascularization.
- Zone III retinal vascularization without previous zone I or II ROP.
- Regression of ROP.
- 50 weeks postmenstrual age with no prethreshold or worse ROP present.
The treatment of ROP
Retinal ablation is the standard treatment of ROP. The laser photo coagulation effectiveness is well established(6). The least severe ROP for which intervention should be considered is type 1 ROP. Ideally, therapy should be performed within 72 hours after the diagnosis of treatable disease based on ophtalmologist recommendation(8). The structural and functional benefits of treatment have been maintained through the 15-year follow-up report. Because no preventive medical treatment has been confirmed for ROP yet, only interventions made during phase 1 of ROP may lessen the disease(11).
Conclusions
The neonatologist must know the groups of infants who should be screened for ROP. The risk of severe ROP was highest in newborns <28 weeks GA or birth weight <1000 g at birth. The neonatologist must recognize those preterm infants who have been treated with oxygen, because they require a first retinal examination at 4-6 weeks of age, in order to identify those who develop ROP(1).
For the prevention of ROP, it is important to know the causes, to use the oxygen terapy cautiously and to make an early diagnosis of ROP.
Bibliografie
2. An International Committee for the Classification of Retinopaty of Prematurity. The International Classification of Retinopaty of Prematurity - Revisied. Arch Ophthalmol. 123: 991-9, 2005.
3. Fierson WM; American Academy of Pediatrics, Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013; 131(1):189-95.
4. Jalali S, Anand R, Kumar H, Dogra MR, Azad R, Gopal L. Programme planning and screening strategy in retinopathy of prematurity. Indian J Ophthalmol. 2003; 51(1):89–99.
5. American Academy of Pediatrics, Section of Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopaty of prematurity. Pediatrics. 2013; 131:189-195.
6. Uparkar M, Sen P, Rawal A, Agarwal S, Khan B, Gopal L. Laser photocoagulation (810 nm diode) for threshold retinopathy of prematurity: a prospective randomized pilot study of treatment to ridge and avascular retina versus avascular retina alone. Int Ophthalmol. 2011; 31(1):3–8.
7. Vinekar A, Gilbert C, Dogra M, Kurian M, Shainesh G, Shetty B, Bauer N. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014; 62(1):41–9.
8. Early Treatment of Retinopathy of Prematurity Cooperative Group. Revised indications for treatment of retinopathy of prematurity: results of early treatment of retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003; 121:1684–96.
9. Canadian Neonatal Network Annual Reports: http://www.canadianneonatal network.org/portal/ (AccessedDecember 12, 2015).
10. Kennedy KA, Wrage LA, Higgins RD, et al.; SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Evaluating retinopathy of prematurity screening guidelines for 24- to 27-week gestational age infants. J Perinatol 2014; 34(4):311-8.
11. William F. Malcom, et al. Beyond the NICU, Comprehensive Care of the High-Risk infant, 23. Retinopathy of Prematurity and Ophtalmologic Issues, 2015, 347-375.
12. Brodsky D, Martin V. Neonatology Review. 2nd ed. Raleigh, 2010.