Screening for cardiac anomalies represents a critical component of contemporary prenatal care, embodying a proactive approach to identifying congenital heart defects in developing fetuses. Cardiac anomalies represent a diverse spectrum of structural and functional abnormalities that may affect the heart and the great vessels. Prenatal screening for cardiac anomalies is integral to the early detection and management of congenital heart disease. This work is part of a larger study that involved the screening for fetal cardiac anomalies and the journey from suspicion to diagnosis, and analyzed cases screened from 2018 to 2023 at the Prenatal Diagnosis Unit of the University of Medicine and Pharmacy of Craiova, Romania. In this paper, we analyzed certain cases that proved challenging in the first instance or needed a second opinion or an expert examination. We also reviewed cases that were missed in the first trimester and diagnosed later, to establish what was missing from the initial examination to make the diagnosis at that time.
Screening for cardiac anomalies in the first and second trimesters – diagnosing challenging cases
Screeningul anomaliilor cardiace în primul şi al doilea trimestru de sarcină – diagnosticul cazurilor dificile
First published: 30 septembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.41.3.2023.8734
Abstract
Rezumat
Screeningul pentru anomalii cardiace reprezintă o componentă critică a îngrijirii prenatale contemporane, necesitând o abordare proactivă pentru identificarea defectelor cardiace congenitale la feţii în curs de dezvoltare. Anomaliile cardiace reprezintă un spectru divers de anomalii structurale şi funcţionale care pot afecta inima şi vasele mari. Screeningul prenatal pentru anomalii cardiace este parte integrantă a detectării precoce şi a gestionării bolilor cardiace congenitale. Această lucrare face parte dintr-un studiu mai amplu care a implicat screeningul pentru anomalii cardiace fetale şi drumul de la suspiciune la diagnostic, analizând cazurile depistate din 2018 până în 2023 la Unitatea de diagnostic prenatal a Universităţii de Medicină şi Farmacie din Craiova. În această lucrare am analizat anumite cazuri care s-au dovedit provocatoare în primă instanţă sau care au necesitat o a doua opinie ori o examinare suplimentară. Am trecut în revistă şi cazurile care au fost omise în primul trimestru şi diagnosticate ulterior, pentru a stabili ce lipsea de la examinarea iniţială pentru a pune diagnosticul la acel moment.
Introduction
Screening for cardiac anomalies represents a critical component of contemporary prenatal care, embodying a proactive approach to identifying congenital heart defects (CHDs) in developing fetuses. The significance of this screening cannot be overstated, as CHDs stand as the most prevalent congenital birth defects worldwide and constitute a leading cause of neonatal morbidity and mortality(1,2). The multifaceted implications of cardiac anomalies extend beyond the realm of medical care, encompassing psychological, emotional and socioeconomic dimensions for both the affected families and the healthcare system at large. Thus, understanding the importance of prenatal screening for cardiac anomalies requires a comprehensive examination that traverses the medical, ethical and societal dimensions of this practice.
Cardiac anomalies represent a diverse spectrum of structural and functional abnormalities that may affect the heart and great vessels(3). Prenatal screening for cardiac anomalies is integral to the early detection and management of CHDs. The heart, as the central organ responsible for maintaining circulatory function, plays a fundamental role in the survival and well-being of an individual. Congenital heart defects encompass a wide spectrum of conditions, ranging from minor anatomical variations to life-threatening malformations. The detection of cardiac anomalies during the prenatal period assumes a paramount importance(3). The timely diagnosis through prenatal screening allows for early intervention, enabling healthcare providers to initiate appropriate medical care or surgical interventions promptly after birth. This can significantly improve the prognosis and overall quality of life for affected individuals, reducing the burden on the healthcare system in terms of long-term care and interventions(4).
This paper aims to explore the pivotal role of prenatal screening in identifying cardiac anomalies during gestation and its implications for clinical decision-making and long-term prognosis.
Materials and method
This work is part of a larger study that involved the screening for fetal cardiac anomalies and the journey from suspicion to diagnosis, in analyzed cases screened from 2018 to 2023 at the Prenatal Diagnosis Unit of the University of Medicine and Pharmacy of Craiova, Romania.
In this paper, we analyzed certain cases that proved challenging in the first instance or needed a second opinion or an expert examination. We also reviewed cases that were missed in the first trimester and diagnosed later, to establish what was missing from the initial examination to make the correct diagnosis at that time.
Case series
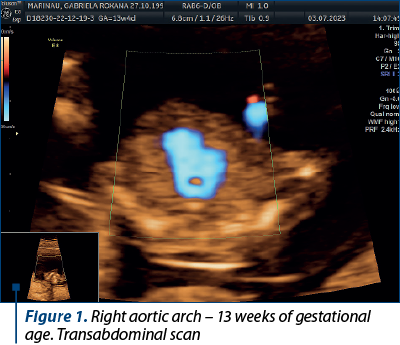
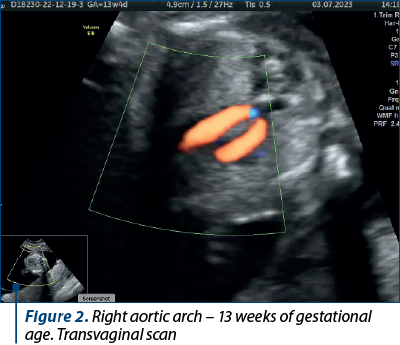
Case 1. Right aortic arch – diagnosis in the first trimester
Congenital anomalies of the aortic arch are diverse and can manifest in various forms, one of which is the right aortic arch (RAA), being characterized by an anomalous right-sided aortic arch that mirrors the typical branching pattern of the aortic arch vessels, potentially leading to compression of the trachea and esophagus. Although RAA is relatively rare(5), it is crucial to diagnose it early, particularly during the first trimester of pregnancy, as it may be associated with other congenital anomalies and genetic syndromes(6). Early diagnosis allows for informed prenatal counseling and preparation for postnatal care.
The primary diagnostic tool for detecting RAA in the first trimester is ultrasound imaging. High-resolution transabdominal or transvaginal ultrasound can visualize the fetal heart and aortic arch, allowing for the identification of abnormal arch anatomy. RAA can be suspected if the three-vessel and trachea (3VT) view shows an unusual right-sided orientation of the aortic arch, with the left common carotid artery arising from it(7).
Achieving a definitive diagnosis of RAA in the first trimester can be challenging, due to the limited fetal size and positioning. Some cases may require follow-up imaging in the second trimester for confirmation. Therefore, it is essential for healthcare providers to communicate effectively with the expectant parents about the possibility of a provisional diagnosis and the need for subsequent evaluations (Figures 1 and 2).
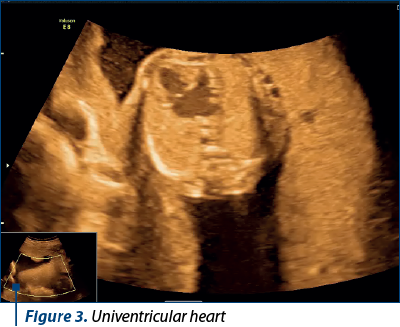
Case 2. Complex malformation, including univentricular heart, missed at the first-trimester scan
Complex cardiac malformations, often involving univentricular hearts, present a significant diagnostic challenge during prenatal screening. We present a case of a complex cardiac malformation, specifically a univentricular heart, that was missed during the first-trimester scan at 12 weeks of gestation. The initial ultrasound examination, while focused on routine screening for fetal anatomy, did not reveal any overt cardiac anomalies.
Univentricular heart (UVH) encompasses a spectrum of complex congenital cardiac defects in which one of the ventricles is underdeveloped or nonfunctional. Timely prenatal diagnosis of UVH has emerged as a critical component of comprehensive prenatal care(3).
While challenges in early detection persist, advancements in diagnostic modalities have significantly improved the accuracy of UVH diagnosis during pregnancy (Figure 3).
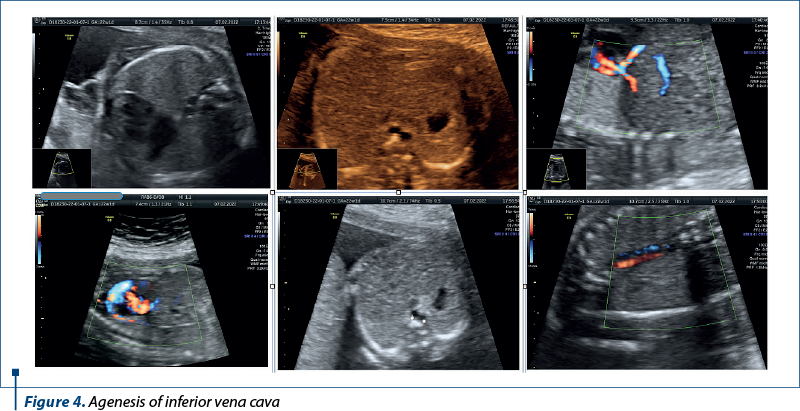
Case 3. Agenesis of inferior vena cava
Agenesis of the inferior vena cava (AIVC) is a rare congenital vascular anomaly that poses unique challenges for prenatal diagnosis and management.
Agenesis of the inferior vena cava is a congenital anomaly characterized by the partial or complete absence of the inferior vena cava, the major venous vessel responsible for returning deoxygenated blood from the lower half of the body to the right atrium of the heart(8). While often asymptomatic in childhood, AIVC may lead to thrombotic complications and deep venous thrombosis in adulthood(9). The prenatal diagnosis of AIVC is challenging, due to its rarity and the evolving nature of fetal venous development (Figure 4).
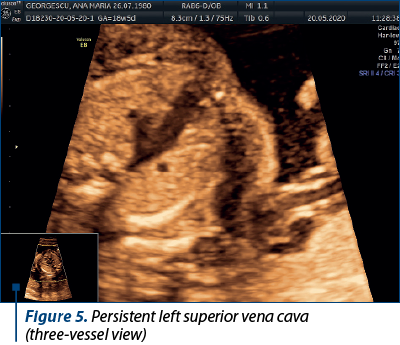
Case 4. Persistence of left superior vena cava
The superior vena cava (SVC) is a critical vessel responsible for returning deoxygenated blood from the upper body to the right atrium of the heart. In most individuals, the right superior vena cava (RSVC) is the dominant and solely persistent vessel. However, in some cases, the left superior vena cava (LSVC) persists alongside the RSVC, resulting in a congenital vascular anomaly(10).
The embryological development of the venous system involves the formation of several paired veins, including the left and right anterior cardinal veins. During normal development, the left anterior cardinal vein regresses, while the right anterior cardinal vein forms the RSVC. When the left anterior cardinal vein fails to regress, it persists as the LSVC. The presence of LSVC is often associated with an absent or small left innominate vein(10). The rarity of LSVC often results in diagnostic challenges.
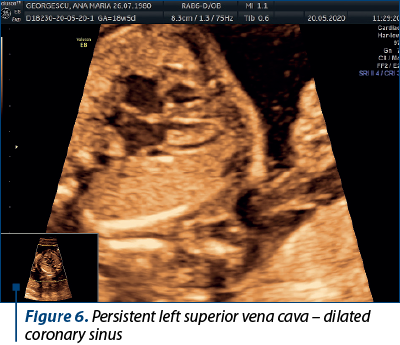
In this particular case, the persistent left superior vena cava (seen along the RSVC in Figure 5) drained in the coronary sinus, and we can notice an enlarged coronary sinus (Figure 6).
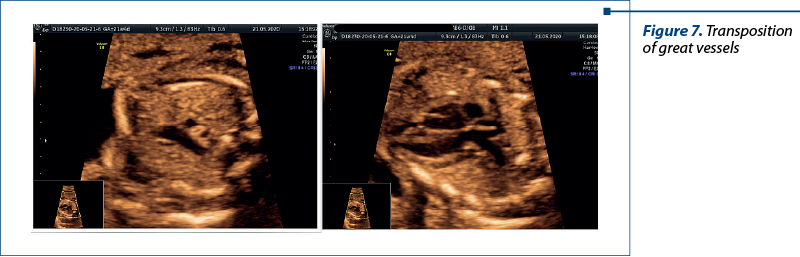
Case 5. Transposition of great vessels – one we should never miss
Transposition of the great vessels (TGV), also known as dextro-transposition of the great arteries (d-TGA), is a critical congenital heart defect that requires prompt intervention after birth(3). Prenatal diagnosis of TGV is crucial for informed decision-making, facilitating timely postnatal care and improving the overall prognosis for affected infants.
Fetal echocardiography is the cornerstone of prenatal TGV diagnosis. High-resolution ultrasound imaging allows for the detailed examination of fetal cardiac anatomy, including the positions of the aorta and pulmonary artery. Specific features, such as “parallel” great vessels and an abnormal relationship between the ventricles, can raise the suspicion of TGV(11,12).
Color Doppler imaging enhances the diagnostic accuracy of fetal echocardiography by visualizing blood flow patterns within the heart and great vessels. It aids in confirming abnormal connections and blood flow patterns characteristic of TGV(13).
Prenatal diagnosis of transposition of the great vessels is a critical component of comprehensive prenatal care. While challenges in early detection persist, advances in fetal echocardiography and color Doppler imaging have significantly improved the diagnostic accuracy(14). Early diagnosis empowers healthcare providers to offer timely interventions, informed parental counseling, and optimal postnatal care planning.
The abnormal three vessels view and the emergence of the pulmonary artery from the left ventricle were critical for the diagnosis (Figure 7).
Discussion
The main issue about the diagnosis in the first trimester, or the second for that matter, is the correct use of standard images recommended in the guidelines. Some special situations (mostly, rare pathology) need additional images like bicaval view or the aortic or ductal arch. The other important step is the use of color Doppler which is a fantastic adjuvant tool to the ultrasound examination of the fetal heart and can help us in many difficult cases. When in doubt, one should always ask for a second opinion.
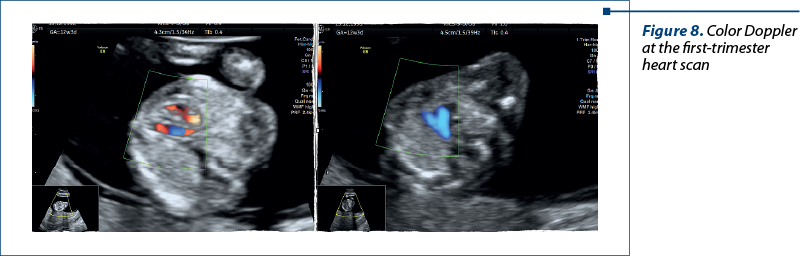
The views that helped us the most in our diagnosis were:
-
In the first trimester – atrial-ventricular flow and three-vessel view with color Doppler.
-
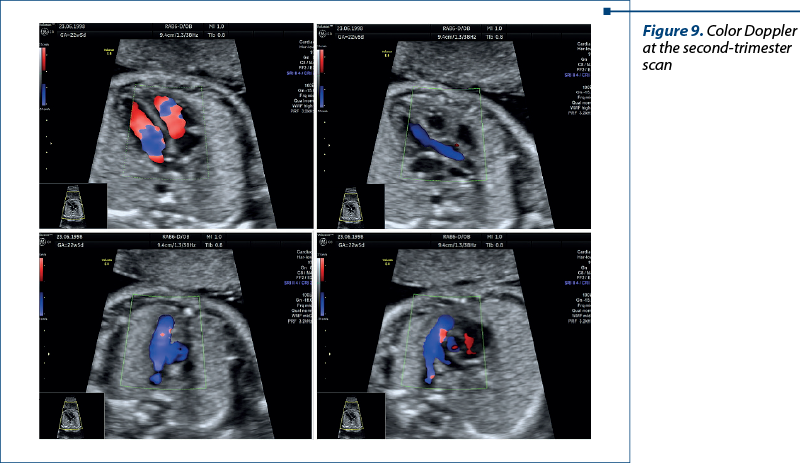
In the second trimester – four-chamber view, five-chamber view, three-vessel view, and short-axis view (with Doppler). In special situations, we used bicaval view and examinations of the arches for a correct diagnosis (Figures 8 and 9).
Studies have consistently shown that early detection and intervention for CHDs are associated with improved outcomes, reduced morbidity and enhanced survival rates(1).
Bakker et al.(15) report an increasing detection rate of CHD after prenatal screening. The increasing prenatal diagnostic rate of CHD is a notable development in the field of perinatal medicine, offering several clinical implications, such as the aforementioned: early detection due to advanced imaging techniques, such as fetal echocardiography and color Doppler ultrasound; improved clinical outcomes in infants with known CHDs who undergo early surgical or medical interventions and have improved clinical outcomes compared to those diagnosed later in life. Early diagnosis enables healthcare providers to plan for specialized delivery, neonatal interventions and postoperative care, ultimately leading to reduced morbidity and mortality rates.
Fetal echocardiography(16-18) is the gold standard for prenatal cardiac screening. It offers high-resolution imaging of fetal cardiac structures and blood flow patterns, allowing for the detection of various CHDs.
Color Doppler(17) imaging complements fetal echocardiography by visualizing blood flow patterns within the fetal heart and great vessels, aiding in the diagnosis of anomalies. The integration of color and pulsed-wave Doppler ultrasound into prenatal cardiac screening has transformed the assessment of blood flow patterns and velocities within the fetal heart. Doppler imaging provides crucial information about the direction and speed of blood flow, allowing for the identification of abnormal flow patterns associated with CHDs.
The accuracy of prenatal cardiac screening may vary depending on the gestational age. Early in pregnancy, fetal size and positioning can limit the visibility of cardiac structures.
Advancements in imaging technology(19,20), such as three-dimensional (3D) and four-dimensional (4D) ultrasound, have improved the diagnostic accuracy of prenatal cardiac screening. 3D and 4D ultrasound technologies provide three-dimensional images and real-time motion sequences of the fetal heart. This advancement allows for a comprehensive evaluation of cardiac structures. Clinicians can rotate and manipulate these images to gain a better understanding of complex cardiac anomalies, facilitating early detection and treatment planning.
Conclusions
Prenatal screening for cardiac anomalies represents a crucial aspect of modern prenatal care with far-reaching consequences. Its significance extends from the immediate medical benefits of early detection and intervention to the ethical, psychological and societal dimensions of family and healthcare system dynamics.
Despite challenges associated with fetal size and complexity of anomalies, recent advancements in diagnostic modalities and algorithms have improved the accuracy of prenatal cardiac screening. This underscores the importance of continuous research and clinical experience in refining prenatal cardiac screening techniques and in optimizing outcomes for infants born with congenital heart defects. As our understanding of cardiac anomalies and prenatal screening techniques continues to evolve, a comprehensive examination of its importance becomes increasingly vital to ensure the best possible care provided for both the unborn child and the expectant parents.
Conflict of interest: none declared.
financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Meller C, Grinenco S, Aiello H, et al. Congenital heart disease, prenatal diagnosis and management. Arch Argent Pediatr. 2020;118(2):e149-e161.
-
Hopkins MK, Dugoff L, Kuller JA. Congenital heart disease: prenatal diagnosis and genetic associations. Obstet Gynecol Surv. 2019;74(8):497-503.
-
Bravo-Valenzuela NJ, Peixoto AB, Araujo Júnior E. Prenatal diagnosis of congenital heart disease: A review of current knowledge. Indian Heart J. 2018;70(1):150-64.
-
Hunter LE, Simpson JM. Prenatal screening for structural congenital heart disease. Nat Rev Cardiol. 2014;11(6):323-34.
-
Topbas Selcuki NF, Senol G, Esin D, et al. Prenatal diagnosis and postnatal outcomes of right aortic arch anomalies. Arch Gynecol Obstet. 2022;306(3):745-52.
-
Vigneswaran TV, Jabak S, Syngelaki A, et al. Prenatal incidence of isolated right aortic arch and double aortic arch. J Matern Fetal Neonatal Med. 2021;34(18):2985-90.
-
Yerlikaya G, Efetürk T, Springer S, Reischer T. Prenatal detection of right aortic arch. Arch Gynecol Obstet. 2019;299(4):933-8.
-
Bronshtein M, Khatib N, Blumenfeld Z. Prenatal diagnosis and outcome of isolated interrupted inferior vena cava. Am J Obstet Gynecol. 2010;202(4):398.e1-398.e4.
-
Dougherty MJ, Calligaro KD, DeLaurentis DA. Congenitally absent inferior vena cava presenting in adulthood with venous stasis and ulceration: A surgically treated case. J Vasc Surg. 1996;23(1):141-6.
-
Keleş A, Yılmaz O, Dağdeviren G, Çelik ÖY, Yücel A, Şahin D. Persistent left superior vena cava: why is prenatal diagnosis important? Fetal Pediatr Pathol. 2022;41(4):592-602.
-
Martins P, Castela E. Transposition of the great arteries. Orphanet J Rare Dis. 2008;3(1):27.
-
Bravo-Valenzuela NJ, Peixoto AB, Araujo Júnior E. Prenatal diagnosis of transposition of the great arteries: an updated review. Ultrasonography. 2020;39(4):331-9.
-
Chaoui R, Bollmann R. Die fetale farbdoppler-echokardiographie - teil 2: fehlbildungen des herzens und der großen gefäße. Ultraschall Med. 2008;15(03):105-11.
-
Bravo-Valenzuela NJ, Peixoto AB, Araujo Júnior E, Da Silva Costa F, Meagher S. The reverse boomerang sign: a marker for first-trimester transposition of great arteries. J Matern Fetal Neonatal Med. 2019;32(4):677-80.
-
Bakker MK, Bergman JEH, Krikov S, et al. Prenatal diagnosis and prevalence of critical congenital heart defects: an international retrospective cohort study. BMJ Open. 2019;9(7):e028139.
-
Behera SK, Ding VY, Chung S, Tacy TA. Impact of fetal echocardiography comprehensiveness on diagnostic accuracy. J Am Soc Echocardiogr. 2022;35(7):752-761.e11.
-
Chaubal NG, Chaubal J. Fetal echocardiography. Indian J Radiol Imaging. 2009;19(01):60-8.
-
McBrien A, Hornberger LK. Early fetal echocardiography. Birth Defects Res. 2019;111(8):370-9.
-
Sachdeva S, Gupta SK. Imaging modalities in congenital heart disease. Indian J Pediatr. 2020;87(5):385-97.
-
Charakida M, Pushparajah K, Simpson J. 3D echocardiography in congenital heart disease: a valuable tool for the surgeon. Future Cardiol. 2014;10(4):497-509.