Perinatal stroke is a polymorphic syndrome caused by a cerebral vascular injury. It occurs between 20 weeks of gestation and the first month of postnatal life, the highest risk being near birth. Perinatal stroke includes a variety of lesions, depending on the vessel affected, the time of diagnosis and the type of lesion. Cumulative maternal, fetal and intrapartum risk factors determine the complex etiopathogenic mechanism. Stroke can be detected early in the neonatal period or in childhood. The symptoms can be easily overlooked, and imaging investigations are relevant for diagnosis and prognosis. The management of acute stroke and the neuroprotective treatment are essential to prevent the adverse effects on the immature brain. Perinatal brain damage has effects on motor and cognitive function, affecting the quality of life.
Accidentul vascular cerebral perinatal – coşmarul neurodezvoltării fetale
Perinatal stroke – a neurodevelopmental nightmare
First published: 12 iulie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.2.2022.6678
Abstract
Rezumat
Accidentul vascular cerebral perinatal este un sindrom polimorf cauzat de o leziune vasculară cerebrală. Apare între 20 de săptămâni de gestaţie şi prima lună de viaţă postnatală, cel mai mare risc fiind în apropierea naşterii. Accidentul vascular cerebral perinatal include o varietate de leziuni, în funcţie de vasul afectat, de momentul diagnosticării şi de tipul leziunii. Factorii de risc cumulativi materni, fetali şi cei intrapartum determină mecanismul etiopatogenic complex. Accidentul vascular cerebral poate fi depistat precoce în perioada neonatală sau în copilărie. Simptomele pot fi uşor trecute cu vederea, iar investigaţiile imagistice sunt relevante pentru diagnostic şi prognostic. Managementul accidentului vascular cerebral cu tratament acut şi neuroprotector este esenţial pentru a preveni efectele adverse asupra creierului imatur. Leziunile cerebrale perinatale au efecte asupra funcţiei motorii şi cognitive, afectând calitatea vieţii.
1. Introduction
Perinatal stroke brings together a series of focal neurological lesions that occur during the development of the fetal brain, with sometimes major repercussions in adult life. The incidence of perinatal stroke is 63 per 100,000 live births, being a major cause of neurological impairment, especially childhood epilepsy(1). Other studies have shown that the incidence of perinatal stroke is estimated to be between 1:1000 and 1:3000 live births and is often underdiagnosed(2,3).
Stroke most commonly affects the left hemisphere (73%) and often involves middle cerebral artery. The etiopathogenic mechanism is caused by the interruption of blood flow due to perinatal ischemic arterial stroke (PAIS) and hemorrhagic stroke. PAIS is common in approximately 70% of perinatal strokes. Another condition is cerebral synovial thrombosis (CSVT), which is less common, while nontraumatic hemorrhagic stroke disrupts the neurological function due to nontraumatic intracerebral hemorrhage. Cases in which the pathophysiological mechanism is not discovered are classified as idiopathic(4,5).
The timing of symptoms differs from the first postnatal days (ischemic stroke, neonatal arterial hemorrhage, cerebral synovial thrombosis) to the end of the first year regarding the characteristic of focal neurological lesions (perinatal ischemic stroke, periventricular venous infarction). The sequelae are represented by hemiparesis, cognitive changes, epilepsy and behavioral and language disorders, their intensity being dependent on the time of onset and the extent of the stroke(6,7).
In recent years, neonatal intensive care has made significant progress. However, perinatal stroke continues to be a major public health problem as it is associated with high morbidity and mortality, including in adulthood. Complications include high rates of epilepsy, cerebral palsy and behavioral disorders. Most patients who survive have a degree of disability throughout their lives, which will be a real problem for both the family and the public health services. Thus, although perinatal stroke is an important factor in morbidity, we find little data on etiopathogenesis, which causes some limitations in terms of prophylaxis, therapeutic management and prognosis of these patients(8).
In the last decade, prenatal and especially postnatal brain imaging has made obvious advances. The prenatal suspicion of stroke is raised by ultrasound examination and supplemented with MRI. The postnatal diagnosis is confirmed by MRI and CT examination that allows the brain's morphometric study, vascularity evaluation and brain metabolism. The symptoms of perinatal stroke may be acutely characterized by seizures or hemiparesis in infants(8).
2. Pathogenesis and risk factors
for perinatal stroke
The etiopathogenic mechanism of perinatal stroke differs depending on the responsible risk factors. Hypoxia occurs in stroke in the affected tissue, which causes significant vasodilation in the immature brain tissue. In regions with increased metabolic activity in the brain, significant vasodilation is performed to temporarily compensate for hypoxemia. If the hypoxia is not corrected and irreversible damage occurs, tissue acidosis and conversion to anaerobic metabolism occur(3).
Presumptive risk factors associated with perinatal stroke are maternal, fetal or intrapartum (Table 1), and the risk increases when these factors are cumulative. Most often, risk factors include uncertain fetal status, neonatal intensive care, fetal heart rate abnormalities, IUGR, Apgar score less than 7 at 5 minutes, emergency cesarean section and prolonged labor. To a lesser extent, preeclampsia, gestational diabetes, nulliparity and the male sex are also responsible. Preeclampsia by decreasing placental blood flow leads to fetal cerebral hypoperfusion and thus the possibility of embolism or ischemic lesions. Another possible cause would be instrumental birth or not associated with fetal distress through exercise performed by direct brain trauma. Superficial trauma is not a cause of neonatal arterial ischemic stroke(3,9,10). Congenital heart disease is another risk factor for neonatal ischemic arterial stroke and for its recurrence(11,12,13).
Prothrombotic abnormalities in both the newborn and the mother, including heterozygous Leiden factor V, prothrombin gene mutation 20210, increased antiphospholipid antibodies and protein C and S deficiency, are associated with perinatal stroke due to a cerebral thrombotic or thromboembolic mechanism(9). Infections can also lead to perinatal stroke. Clinical chorioamnionitis can cause umbilical vein thrombosis(14). Bacterial meningitis (especially with group B Streptococcus) may be associated with arterial ischemic stroke, so intravenous antibiotic treatment should be initiated promptly(3).
There has recently been evidence of placental thromboembolism as a possible cause of neonatal ischemic arterial stroke(15).
3. Classification of perinatal stroke
Perinatal stroke can be classified by the timing of diagnosis, the vessels involved and the type of injury. The timing of diagnosis may be in the acute neonatal period or retrospectively after a period of normal development followed by abnormal neurologic findings, with the injury presumed to have occurred around the time of birth. Strokes may be arterial or venous, ischemic and/or hemorrhagic. Based on clinical and radiographic features, six perinatal stroke diseases are highlighted (Figure 1).
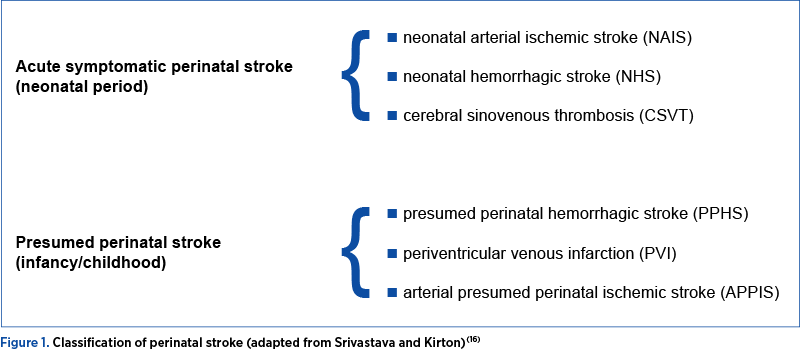
3.1 Neonatal arterial ischemic stroke (NAIS)
It has an increased incidence of 6 to 17/100,000, being found in over 90% of cases, especially near term and rarely in premature infants. Symptoms begin at 12-72 hours after birth with focal or generalized seizures. The baby may also experience dizziness, encephalopathy, cortical hyperexcitability, swallowing disorders and decreased muscle tone(7).
The etiopathogenesis of NAIS is dominated by the obliteration of the internal carotid artery, hypoxia-ischemia peripartum, or the phenomena of antenatal infection or inflammation. The main risk factor for NAIS is perinatal inflammation induced by the infectious process in the placenta, brain and cerebral artery walls. Knowledge of these phenomena is particularly important in developing vascular and neuroprotective anti-inflammatory substances(17). Chevin et al., in a preclinical study, highlighted the neuroprotective role in NAIS of therapeutic hypothermia in the diffuse processes of cerebral hypoxia-ischemia in neonatal encephalopathy(18).
Imaging examination by diffusion MRI presents a focal area secondary to infarction comprising one or more ischemic arterial territories, especially in the middle cerebral artery, mainly on the left side. The mechanism is of the occlusive type, the arterial vascular lesions being rare. Stroke volume analysis performed by acute diffusion-weighted imaging can be used to analyze the cerebral palsy of newborns(19).
3.2 Neonatal cerebral sinovenous thrombosis (CSVT)
It is a rare condition in neonates (with an incidence of 0.6 per 100,000) that involves the identification of thrombi in the cerebral veins or dural sinuses(20). The consequence is a parenchymal venous infarction of hemorrhagic cause in the thalamus with intraventricular extension, which dominates the clinical picture regarding the diagnosis of convulsions in the first days of life. MRI and venography may show an infarction, venous congestion and a cerebral venous filling defect(21).
The main incriminating risk factors are infection and mechanical compression syndrome of the sinuses. Low molecular weight or unfractionated anticoagulant therapy is considered safe, with some delays in the presence of bleeding. The positive effect of this therapy is to reduce the progression of the thrombus and promote complete recanalization of the vessels, leading to a reduction in morbidity. CSVT is characterized by increased morbidity and mortality(21,22).
3.3 Neonatal hemorrhagic stroke (NHS)
It is a condition specific to children born at term and represents a focal accumulation of blood in the cerebral parenchyma, which leads to the installation of encephalopathy, neurological deficit, convulsions and hypotonia. The incidence of neonatal hemorrhagic stroke is 1:6300 live births, while idiopathic intracranial hemorrhage affects 1:9500 live births(23).
MRI angiography and MRI venography performed on newborns show thin areas underlying the vessel wall, the temporal lobe’s most common location. The most common topography of the NHS is the temporal lobe; the recurrence of the condition is rare and is associated with devastating neurodevelopmental outcomes(23,24).
3.4 Arterial presumed perinatal ischemic stroke (APPIS)
With an estimated incidence of 1:3600 live births, this condition is suspected in children below 1 year of age who have hemiparesis, early motor asymmetry or handedness, and less frequently between 3 and 10 years of age when it is manifested. Possible perinatal arterial damage of ischemic stroke may have an MRI representation, therapeutic management based on rehabilitation and recovery methods of motor deficits, and cognitive and behavioral disorders. The risk of ischemic stroke recurrence after a suspected perinatal stroke is low, and in the absence of guidelines, routine thrombophilia screening is indicated for these patients to benefit from secondary prophylaxis(25). Bektaş et al. found that more than 50% of APPIS cases will develop childhood epilepsy, usually drug-resistant(26).
3.5 Periventricular venous infarction (PVI)
The diagnosis of this condition is made in children born at term and is characterized by the presence of congenital hemiparesis objectified by highlighting lesions on MRI(27).
The bleeding gives etiopathogenesis in the germinal matrix before 34 weeks of gestation, which leads to a compressive phenomenon in the adjacent cerebral veins at the level of the infarct area of the white periventricular substance. The incidence is unknown because the diagnosis is made late based on the asymmetric motor deficit, on average at 18 months. Radiological imaging investigations and primarily MRI show unilateral enlargement of the lateral ventricle with porencephaly. Possible risk factors include maternal hypertension, recurrent miscarriage, antepartum bleeding and prenatal infection. The treatment is strictly for motor rehabilitation(28,29).
3.6 Presumed perinatal hemorrhagic stroke (PPHS)
The lesion develops after the first 28 days of life, similar to the presumed perinatal ischemic stroke. The risk factors include genetic conditions such as hereditary hemorrhagic telangiectasia, hemophilia or acquired hemorrhagic diathesis, such as immune thrombocytopenia. In addition, the small presumed perinatal hemorrhagic stroke could appear asymptomatic outside the perinatal period in early childhood and can be detected only on subsequent imaging. The symptoms are represented by convulsions, early motor asymmetry and developmental delay, and MRI makes the diagnosis. Therefore, the treatment is directed at the clinical symptoms specific to epilepsy(16).
4. Diagnosis
The symptoms depend on the onset and intensity of perinatal stroke. In the neonatal period, usually in the first three days of life, the symptoms include focal seizures, tone abnormality, feeding difficulty, apnea, fever, chewing, or cycling movements and irritability. In rare cases, progressive hemiplegia may occur. Later in the first year of life, symptoms such as hemiplegia, early pathological hand, spasticity and delayed neuronal development may occur. Motor impairment or epilepsy affects half to two-thirds of newborns with a stroke(7,30,31).
The diagnosis following clinical suspicion is confirmed by imaging. Cranial ultrasound is usually performed on the first line and can detect specific lesions, and by using Doppler examination, brain perfusion can be assessed. Computed tomography (CT) has low sensitivity in the acute phase and is radiant, therefore it is not recommended. MRI defines the type of stroke, identifies the damaged regions, can measure various aspects of brain development and, in addition, is useful for the prognosis. The examination should be completed with MR angiography of the cerebral and neck arteries. In most cases, arterial dissection cannot be objectified by MR angiography, being highlighted pathologically post-mortem. Diffusion weight imaging (DWI) is recommended at the onset of symptoms, showing restricted diffusion early. It is preferred to postpone the MRI or to repeat it after a few days in order to correctly target the localization and extension of the infarction. MR venography (MRV) is indicated for the diagnosis of sinus venous thrombosis(30,31).
5. Therapeutic management –
general considerations
The therapeutic acute management is based on neuroprotection and emergency vascular repermeabilization. Anticoagulants can be used in severe thrombophilia or synovial thrombosis, without intracranial hemorrhage, for 6-12 weeks, as the drug itself has a risk of hemorrhage. Imaging in infants/children with intracranial hemorrhage treated with anticoagulants for one week showed an increase in the size of clots(3,8).
An electroencephalogram (EEG) is recommended to detect seizures and assess the response to treatment. In the case of seizures, anticonvulsant therapy (phenobarbital) is associated for a short time, to avoid the neurotoxic effects of antiepileptics on the immature brain(16). Regarding the neuroprotective approach, hypothermia can reduce the number of seizures in newborns. It is important to maintain homeostasis by administering oxygen and maintaining adequate hydration and normoglycemia(31).
The hematological examination of the newborn is important in determining the value of hemoglobin and the possible need for transfusion. Surgery to remove the hematoma or acute decompressive craniectomy is performed in hemorrhagic stroke complicated by compression of the brainstem or hernia with massive hemorrhage(31,32).
For children with cerebral palsy, rehabilitation of motor function is an important element in the recovery of these patients. Other therapeutic means include neuromuscular electrical stimulation and observation of the action. Therapeutic neuromodulation is effective in noninvasive neurostimulation in children with hemiparesis. Repetitive transcranial magnetic stimulation is effective for the motor cortex in children with perinatal stroke and hemiparetic cerebral palsy(7).
6. Neurological impairments of perinatal stroke
The consequences of perinatal stroke can be serious, with several patients being left with neurological sequelae throughout their lives.
Perinatal stroke often leads to disabilities through impaired motor development, dependence on topography, number and size of infarction areas. The clinical symptoms are due to the presence of hemiparesis affecting both the arms and the legs. The study conducted by Kirton found that, in neonatal stroke, motor deficits are present in 50-60% and in the case of perinatal stroke, in 80-90%. The main objective is to recover the motor sequelae on an outpatient basis, especially at the level of the upper extremities(33,34).
The incidence of epilepsy in patients with seizures after neonatal arterial ischemic stroke varies between 16.4%(35) and 27.2%(36). The identification and treatment of seizures in children with perinatal stroke resulted in a decrease in the incidence of epilepsy that was associated with poor neuronal development. Epileptic encephalopathy may be suspected in case of changes in neurodevelopment, which is why the electroencephalogram is indicated. In cases of stroke with cortical involvement, the cognitive function may be impaired as the child grows(1,31).
7. Conclusions
In recent years, the estimated risk of perinatal stroke has been increasing at birth. This requires new research to understand etiopathogenesis and define the therapeutic management and prophylactic methods. The perinatal stroke should be diagnosed early to reduce sequelae by appropriate neuroprotective treatment. Etiopathogenesis is sometimes difficult to establish in all forms of perinatal stroke, and this is especially important in terms of efforts for the neurorehabilitation of brain function and to reduce the incidence of mental health problems in patients during their lifetime.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Laugesaar R, Vaher U, Lõo S, et al. Epilepsy after perinatal stroke with different vascular subtypes. Epilepsia Open. 2018;3(2):193-20. https://doi.org/10.1002/epi4.12104.
-
Srivastava R, Kirton A. Perinatal Stroke: A Practical Approach to Diagnosis and Management. Neoreviews. 2021;22(3):e163-e176. doi:10.1542/neo.22-3-e163.
-
Whitaker EE, Cipolla MJ. Perinatal Stroke. Handb Clin Neurol. 2020;171:313–326. https://doi.org/10.1016/B978-0-444-64239-4.00016-3.
-
Kirton A, Armstrong-Wells J, Chang T, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128(6):e1402-e1410. https://doi.org/10.1542/peds.2011-1148.
-
Srivastava R, Kirton A. Perinatal Stroke: A Practical Approach to Diagnosis and Management. NeoReviews. 2021;22(3):e163–e176. https://doi.org/10.1542/neo.22-3-e163.
-
deVeber GA, Kirton A, Booth FA, et al. Epidemiology and Outcomes of Arterial Ischemic Stroke in Children: The Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol. 2017;69:58-70. https://doi.org/10.1016/j.pediatrneurol.2017.01.016.
-
Dunbar M, Kirton A. Perinatal Stroke: Mechanisms, Management, and Outcomes of Early Cerebrovascular Brain Injury. Lancet Child Adolesc Health. 2018;2(9):666–676. https://doi.org/10.1016/S2352-4642(18)30173-1.
-
Dunbar M, Kirton A. Perinatal Stroke. Semin Pediatr Neurol. 2019;32:100767. https://doi.org/10.1016/j.spen.2019.08.003.
-
Mineyko A, Kirton A. The Black Box of Perinatal Ischemic Stroke Pathogenesis. J Child Neurol. 2011;26 (9):1154–1162. https://doi.org/10.1177/0883073811408312.
-
Fernández-López D, Natarajan N, Ashwal S, Vexler ZS. Mechanisms of Perinatal Arterial Ischemic Stroke. J Cereb Blood Flow Metab. 2014;34(6):921–932. https://doi.org/10.1038/jcbfm.2014.41.
-
Asakai H, Cardamone M, Hutchinson D, et al. Arterial ischemic stroke in children with cardiac disease. Neurology. 2015;85(23):2053-2059. https://doi.org/10.1212/WNL.0000000000002036.
-
Rodan L, McCrindle BW, Manlhiot C, et al. Stroke recurrence in children with congenital heart disease. Ann Neurol. 2012;72(1):103-111. https://doi.org/10.1002/ana.23574.
-
Vázquez-López M, Castro-de Castro P, Barredo-Valderrama E, et al. Ischaemic stroke in children with cardiopathy: An epidemiological study. [Ictus isquémico en niños con cardiopatía: estudio epidemiológico]. Neurologia. 2017;32(9):602-609. https://doi.org/10.1016/j.nrleng.2016.03.007.
-
Dueck CC, Grynspan D, Eisenstat DD, Caces R, Rafay MF. Ischemic perinatal stroke secondary to chorioamnionitis: a histopathological case presentation. J Child Neurol. 2009;24(12):1557-1560. https://doi.org/10.1177/0883073809341271.
-
Bernson-Leung ME, Boyd TK, Meserve EE, et al. Placental Pathology in Neonatal Stroke: A Retrospective Case-Control Study. J Pediatr. 2018;195:39-47.e5. https://doi.org/10.1016/j.jpeds.2017.11.061.
-
Srivastava R, Kirton A. Perinatal Stroke: A Practical Approach to Diagnosis and Management. NeoReviews. 2021;22(3):e163–e176. https://doi.org/10.1542/neo.22-3-e163.
-
Giraud A, Guiraut C, Chevin M, Chabrier S, Sébire G. Role of Perinatal Inflammation in Neonatal Arterial Ischemic Stroke. Front Neurol. 2017;8:612. https://doi.org/10.3389/fneur.2017.00612.
-
Chevin M, Chabrier S, Dinomais M, Bedell BJ, Sébire G. Benefits of hypothermia in neonatal arterial ischemic strokes: A preclinical study. Int J Dev Neurosci. 2020;80(4):257-266. https://doi.org/10.1002/jdn.10022.
-
Wiedemann A, Pastore-Wapp M, Slavova N, et al. Impact of stroke volume on motor outcome in neonatal arterial ischemic stroke. Eur J Paediatr Neurol. 2020;25:97-105. https://doi.org/10.1016/j.ejpn.2019.10.006.
-
Grunt S, Wingeier K, Wehrli E, et al. Cerebral sinus venous thrombosis in Swiss children. Dev Med Child Neurol. 2010;52(12):1145-1150. https://doi.org/10.1111/j.1469-8749.2010.03722.x.
-
Ramenghi LA, Cardiello V, Rossi A. Neonatal Cerebral Sinovenous Thrombosis. Handb Clin Neurol. 2019;162:267–280. https://doi.org/10.1016/B978-0-444-64029-1.00012-6.
-
Herman I, Karakas C, Webber TA, et al. Clinical Profile and Long-Term Outcome in Neonatal Cerebral Sinus Venous Thrombosis. Pediatr Neurol. 2021;121:20-25. https://doi.org/10.1016/j.pediatrneurol.2021.05.001.
-
Cole L, Dewey D, Letourneau N, et al. Clinical Characteristics, Risk Factors, and Outcomes Associated With Neonatal Hemorrhagic Stroke: A Population-Based Case-Control Study [published correction appears in JAMA Pediatr. 2017 Jun 1;171(6):602]. JAMA Pediatr. 2017;171(3):230-238. https://doi.org/10.1001/jamapediatrics.2016.4151.
-
Tan AP, Svrckova P, Cowan F, Chong WK, Mankad K. Intracranial hemorrhage in neonates: A review of etiologies, patterns and predicted clinical outcomes. Eur J Paediatr Neurol. 2018;22(4):690-717. https://doi.org/10.1016/j.ejpn.2018.04.008.
-
Hamilton K, Salman MS, Schwartz I, McCusker PJ, Wrogemann J, Rafay MF. Arterial ischemic stroke in an adolescent with presumed perinatal ischemic stroke. J Child Neurol. 2012;27(1):94-98. https://doi.org/10.1177/0883073811414421.
-
Bektaş G, Kipoğlu O, Pembegül Yıldız E, et al. Epileptic spasm and other forms of epilepsy in presumed perinatal arterial ischemic stroke in Turkey after more than 10 years follow-up: A single centre study. Brain Dev. 2019;41(8):699-705. https://doi.org/10.1016/j.braindev.2019.04.004.
-
Lõo S, Ilves P, Männamaa M, et al. Long-term neurodevelopmental outcome after perinatal arterial ischemic stroke and periventricular venous infarction. Eur J Paediatr Neurol. 2018;22(6):1006-1015. https://doi.org/10.1016/j.ejpn.2018.07.005.
-
Vitagliano M, Dunbar M, Dyck Holzinger S, et al. Perinatal arterial ischemic stroke and periventricular venous infarction in infants with unilateral cerebral palsy. Dev Med Child Neurol. 2022;64(1):56-62. https://doi.org/10.1111/dmcn.15000.
-
Fehlings D, Krishnan P, Ragguett RM, et al. Neurodevelopmental profiles of children with unilateral cerebral palsy associated with middle cerebral artery and periventricular venous infarctions. Dev Med Child Neurol. 2021;63(6):729-735. https://doi.org/10.1111/dmcn.14818.
-
Fernández-López D, Natarajan N, Ashwal S, Vexler ZS. Mechanisms of perinatal arterial ischemic stroke. J Cereb Blood Flow Metab. 2014;34(6):921-932. https://doi.org/10.1038/jcbfm.2014.41.
-
Fluss J, Dinomais M, Chabrier S. Perinatal stroke syndromes: Similarities and diversities in aetiology, outcome and management. Eur J Paediatr Neurol. 2019;23(3):368-383. https://doi.org/10.1016/j.ejpn.2019.02.013.
-
Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of Stroke in Neonates and Children: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51-e96. https://doi.org/10.1161/STR.0000000000000183.
-
Kirton A. Advancing Non-Invasive Neuromodulation Clinical Trials in Children: Lessons from Perinatal Stroke. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2017;21(1):75–103. https://doi.org/10.1016/j.ejpn.2016.07.002.
-
Vuillerot C, Marret S, Dinomais M. [Long term outcome of perinatal stroke]. Arch Pediatr Organe Off Soc Francaise Pediatr. 2017;24(9S):9S51-59S60. https://doi.org/10.1016/S0929-693X(17)30332-9.
-
Suppiej A, Mastrangelo M, Mastella L, et al. Pediatric epilepsy following neonatal seizures symptomatic of stroke. Brain Dev. 2016;38(1):27-31. https://doi.org/10.1016/j.braindev.2015.05.010.
-
Rattani A, Lim J, Mistry AM, et al. Incidence of Epilepsy and Associated Risk Factors in Perinatal Ischemic Stroke Survivors. Pediatr Neurol. 2019;90:44-55. https://doi.org/10.1016/j.pediatrneurol.2018.08.025.
Articole din ediţiile anterioare
Tipuri actuale de naştere şi impactul lor asupra mamei şi fătului
În urma evoluţiei modalităţii de naştere, am constatat, potrivit unui studiu observaţional efectuat în clinica noastră în perioada 2017-2021, o ten...
Sarcina şi naşterea în timpul pandemiei de COVID-19. Implicaţii pentru gravidă şi naştere
In late December 2019, a series of pneumonia cases of unknown cause appeared in Wuhan City, Central China, which were reported to the World Health ...
Myasthenia gravis şi sarcina
Myasthenia gravis (MG) is an autoimmune disorder affecting nearly 1 million individuals worldwide, being diagnosed typically in the second and thir...
Cefalhematomul în obstetrică: o minirevizuire a unei perspective multidisciplinare
Cefalhematomul este o afecţiune benignă care descrie o colecţie hemoragică subperiosteală ce apare la nou-născuţi.