Infertility affects one out of ten reproductive age couples globally, while in Romania one out of six couples is reported to seek infertility counsel. The success of reproduction is highly dependent on maternal-fetal immune interaction. The balance between all activator and inhibitor signals between uterine natural killer (uNK) cells and the trophoblast represents a key factor and may influence embryo implantation. Uterine natural killer cells express surface receptors (KIR) which, after recognition of class I HLA molecules expressed by the trophoblast, may stimulate or inhibit the ability of uNK cells to produce soluble factors and may present a low toxicity required to maintain the embryo and allogeneic fetus. We aimed to prove that the investigation of the genetic background of uNK receptors represented by KIR genotyping may be a useful tool in the assessment of recurrent implantation failure (RIF), may help predict therapy outcome, and clarify disease pathogenesis. This is a prospective study conducted on 96 RIF patients and 80 fertile controls. We aimed to investigate whether the presence or absence of each gene or KIR genotype is correlated to the implantation success or to first-trimester miscarriage. The study results point out that the AA inhibitor genotype is associated with weak results in terms of achieving pregnancy following in vitro fertilization (IVF), and the presence or absence of certain genes is correlated to the success of implantation and to first-trimester miscarriage.
Evaluarea expresiei genei KIR la pacienţii cu eşec recurent al implantării
Evaluation of KIR gene expression in patients with recurrent implantation failure
First published: 31 octombrie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.3.2022.7205
Abstract
Rezumat
Infertilitatea afectează unul din zece cupluri de vârstă reproductivă la nivel global, în timp ce, în România, unul din şase cupluri solicită consiliere pentru infertilitate. Succesul reproducerii depinde în mare măsură de interacţiunea imună materno-fetală. Echilibrul dintre semnalele activatoare şi inhibitoare dintre celulele natural killer uterine (uNK) şi trofoblast reprezintă factorul-cheie şi poate influenţa implantarea embrionului. Celulele uNK exprimă receptori de suprafaţă (KIR), care, după recunoaşterea moleculelor HLA de clasa I exprimate de trofoblast, pot stimula sau inhiba capacitatea celulelor uNK de a produce factori solubili şi pot prezenta o toxicitate scăzută, necesară pentru menţinerea embrionului şi a fătului alogen. Ne-am propus să dovedim că investigarea fondului genetic al receptorilor uNK, reprezentat de genotiparea KIR, poate fi un instrument util în evaluarea eşecului recurent al implantării (RIF), poate prezice rezultatul terapiei şi ajută la clarificarea patogenezei bolii. Acesta este un studiu prospectiv realizat la 96 de paciente cu RIF şi la 80 de paciente fertile. Ne-am propus să investigăm dacă prezenţa sau absenţa fiecărei gene sau genotip KIR este corelată cu succesul implantării sau cu avortul spontan în primul trimestru. Rezultatele studiului subliniază că genotipul inhibitorului AA este asociat cu rezultate slabe în ceea ce priveşte obţinerea sarcinii după fertilizarea in vitro (FIV), iar prezenţa sau absenţa anumitor gene este corelată cu succesul implantării şi cu avortul spontan în primul trimestru.
Introduction
Implantation failure corresponds, from the clinical point of view, to two different situations: one in which there is no biological proof of pregnancy (beta-hCG <5 mUI/ml), and another one in which beta hCG (human chorionic gonadotrophin) levels rise 14 days following the embryo transfer, but the pregnancy does not evolve beyond the stage of ultrasonographic detection of a intrauterine gestational sac.
The most frequently used definition found in literature describes the recurrent implantation failure (RIF) as the incapacity to achieve pregnancy following ≥3 IVF (in vitro fertilization) procedures with good quality embryotransfers(1-3). However, this definition is still under debate since it doesn’t take into consideration the number and stage of development of the embryos transferred.
Recently, another definition was acknowledged, which stated that RIF represents the absence of implantation following two consecutive IVF cycles with either four embryos in the cleavage stage, or at least two blastocyst stage embryos being transferred, provided that all embryos were of good quality and were found in the appropriate stage of development(4).
Nowadays, many researchers investigate the role of uterine natural killer cells (uNK) in normal and pathologic pregnancies, because they represent the dominant immune cell population of the endometrium and come in close contact with extravillous trophoblast cells at the beginning of pregnancy. uNK cells express surface receptors (immunoglobulin type killer-cell receptor – KIR) which, after the recognition of class I HLA (human leukocyte antigen) molecules at the trophoblast level (HLA-C, HLA-G), may stimulate or inhibit the ability of uNK cells to produce soluble factors and may present a low toxicity required to support the development of the embryo and allogeneic fetus in the following stages of pregnancy(5-7).
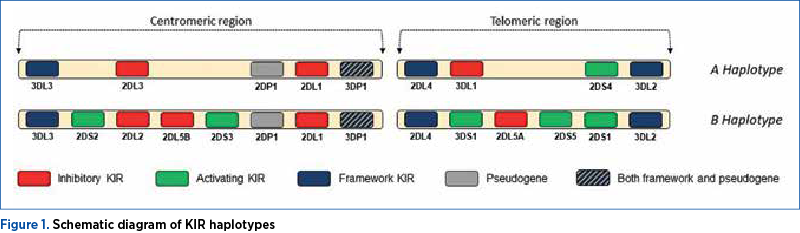
KIR molecules are extremely polymorphic, which means that the sequences of their genes vary largely among individuals, therefore different individuals will present with varying networks/directories of KIR genes. Each individual bears a repertoire composed of some of the 16 KIR genes described which regulate the activity of uNK cells. The KIR gene profile can be divided, according to the KIR genes they contain, into haplotype A and haplotype B. The basis of the two haplotypes is represented by four framework genes: KIR3DL3, at the end of the centromeric region; KIR3DL2, at the end of the telomeric region; KIR3DP1 and KIR2DL4, in the middle. They may also include two pseudogenes: KIR3DP1 (which is also a framework gene) and KIR2DP1(8,9).
Aside from framework genes and pseudogenes, haplotype A may also include the activating KIR2DS4 gene and the KIR2DL1, KIR3DL1 and KIR2DL3 inhibiting genes. The type B haplotype may include one or more activating (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, KIR3DS1) or inhibiting genes (KIR2DL1, KIR2DL2 and KIR2DL5) – Figure 1. Thus, haplotype A is mainly inhibiting, while haplotype B is dominated by activating genes(10). Since each individual presents a pair of the two kinds of haplotypes, the patients can bear three genotypes: AA, AB and BB.
In this context, we may speculate that the success of reproduction partly depends on the maternal immune tolerance of the fetus. The balance between all activating and inhibiting signals between decidual NK cells and the trophoblast represents a key factor and may influence embryo implantation. We believe that the investigation of the genetic background of receptors involved in this process – such as KIR – may aid in the diagnosis of RIF, predict the outcome of assisted reproductive procedures, and may aid in clarifying the pathogenesis of the disease.
Materials and method
In order to test our hypothesis, we designed a prospective study using samples collected from patients managed in the Assisted Reproduction Department of the Obstetrics and Gynecology I Clinic, “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania. All patients enrolled were counseled prior to entering the study and provided their written consent. The study was approved by the Ethics Committee of the “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca.
Plot formation
The study group included 96 Caucasian infertile patients with indication of IVF which addressed the Assisted Reproduction Department of the Obstetrics and Gynecology I Clinic, Cluj-Napoca. The inclusion criteria for the study population were: infertile patients with ages from 18 to 45 years old, with at least two prior IVF procedures followed by the transfer of at least four good quality cleavage stage embryos or two blastocyst stage embryos. The exclusion criteria were the following: patients with atrophic endometrium (<7 mm), metabolic or endocrine disorders, patients with ovarian hyperstimulation syndrome, those with uterine/adnexal pathology (submucous myoma, polyps, unoperated hydrosalpinx, endometriosis etc.). Data regarding infertility work-up and treatment follow-up were collected from the patients’ files. Just prior to the oocyte retrieval, 5 ml of blood were sampled by venipuncture of the cubital vein. Fresh embryo transfers were performed in all cases.
In the control group, we enrolled 80 healthy patients with ages ranging from 18 to 45 years old, with no relevant medical/surgical history, no history of miscarriage, with at least two term live births, and with no pregnancy-associated complications. Patient’s information was retrieved from individual files. For the genetic analysis, we collected 5 ml of venous blood from the cubital vein in the first hours after delivery.
From the blood collected, KIR genes were analyzed and compared between the two groups and their predictive value for pregnancy success was evaluated. The results obtained were analyzed in correlation to pregnancy and first-trimester miscarriage rates.
DNA extraction and KIR typing
The thawed blood sample was centrifuged for 15 minutes at 15,000 rpm and most of the supernatant was removed (for concentration). DNA extraction was performed using EPICENTRE MasterPureTM Complete DNA and RNA Purification Kit® from Illumina company. The concentration and purity of DNA was determined and KIR typing was performed with KIR-Ready Gene® (manufacturer: Inno-train DIAGNOSTIC GMBH, Germany). After DNA amplification and detection on agarose gel by RT-PCR technique, the results were interpreted respecting the manufacturer’s indications. We analyzed all the 16 known KIR genes, the KIR2DL4-del deletion version, the KIR2DS4-del-22bp deletion version, the recombinant version of KIR3DP1 and KIR2DL5-null.
Statistical analysis
Qualitative and quantitative variables were expressed as proportions and means with 95% confidence intervals (95% CI) and odds ratio (OR). The comparison of the two groups was made using the t-Student test in case of quantitative variables, while the Chi-square or two-tailed Fisher Exact tests were employed for qualitative variables. A p-value below 0.05 was considered statistically significant. We calculated correlations between variables, reporting the Pearson coefficient. The analysis of the statistical data was performed using the SPSS Software., version IBM 22.
Results
The mean age of the study group patients was 35±5.1 years old (ranging from 26 to 45 years old), and the Body Mass Index (BMI) varied between 16.7 and 34.6 kg/m2. The mean age of conceptual partners was 37±5.5 years old (range: 23-50 years old), and their sperm count varied between 0.2 and 80x106/ml. The couples had a mean history of infertility of 4.4±2.3 years old. The average oocyte number obtained by ovarian puncture was 5.2±3.4 (range: 1-20), and the average number of embryos was 6 (range: 1-11). In the control group, the average age of the patients included in the study was 35±5.6 years old (range: 18-45 years old), and the BMI ranged from 17.4 to 38.2 kg/m2. There were no statistically significant differences between the patients included in the study group and those included in the control group in terms of age and BMI.
Of the 96 patients enrolled in the study group, 34 (35.41%) achieved biochemical pregnancy (ascertained by positive beta-hCG or urinary test two weeks after the embryo transfer). In twelve pregnancies, there was no fetal cardiac activity at the six-week ultrasound and no first-trimester miscarriage was recorded. Only 22 (22.92%) patients achieved term live births.
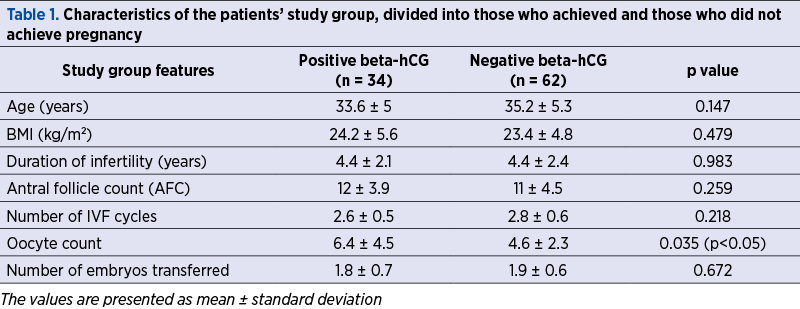
There were no statistically significant differences in the study group between patients who achieved pregnancy or not regarding age, BMI, duration of infertility, antral follicle count, number of embryos obtained, or number of embryos transferred. We noted that the number of oocytes obtained at pick-up was statistically significant lower in patients who didn’t achieve pregnancy as compared to those with a positive pregnancy test (Table 1).
We noticed a significantly increased expression of the KIR2DS1 activating gene in fertile patients from the control group compared to the RIF group patients (OR 0.4887; 95% CI; 0.2492-0.9585; p<0.0372).
Considering the pregnancy rate and the evolution of pregnancy, we obtained significantly statistical results for the KIR3DS1 gene (significantly increased frequency in patients who conceived: r(96)=0.31; p<0,01) and KIR2DL5B(null) (significantly increased frequency in patients who miscarried compared to those who achieved live births: r(34) = -0.46; p<0,01).
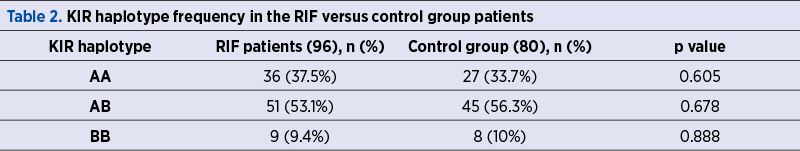
We noticed a reduced frequency of the inhibitory AA haplotype and a greater frequency of the activating AB and BB KIR haplotypes in the control group compared to the RIF group (AA – 33.7%, AB – 56.3%, BB – 10%, compared to AA – 37.5%, AB – 53.1%, BB – 9.4% respectively). However, there were no statistically significant differences between the two groups with regard to the frequency of the three haplotypes (Table 2).
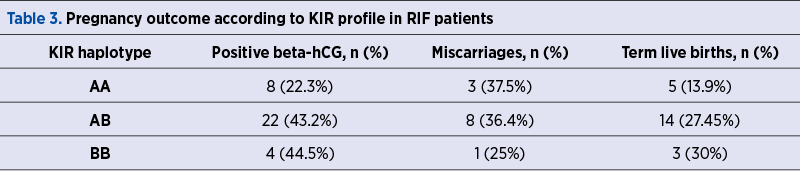
We estimated the pregnancy rates, the miscarriage rates and the live birth rates in RIF patients divided by KIR haplotype and we noted a higher biochemical pregnancy rate in the AB (OR 0.3766; 95% CI; 0.1440-0.9851; p=0.0465) and, respectively, BB haplotype patients (OR 0.3571; 95% CI; 0.0772-1.6522; p =0.0423) compared to AA profile patients. At the same time, there was a larger first-trimester miscarriage rate in AA and AB patients compared to BB profile patients. The results are presented in Table 3.
Discussion
The literature records statistically significant differences between the frequency of KIR genes among populations from different geographical areas, different races and ethnical backgrounds, leading to contradictory results reported by different study teams(11). Our analysis was conducted on Caucasian patients from North-Eastern Romania.
In a recently published research, Yin et al. demonstrated the presence of statistically significant differences in the frequency of KIR2DS1, KIR2DS4, KIR2DS5 and KIR3DL1 gene profiles in populations from various Chinese regions(12). The AA haplotype has a very high prevalence among the Han Chinese population, reaching a rate as high as 50% in certain regions of the country(12,13). Thus, the investigators who only analyzed differences between different ethnic groups and did not take into account the geographical area could reach erroneous conclusions.
Although we obtained a higher frequency of the inhibitory AA genotype and a lower frequency of the activating AB and BB genotypes in RIF patients compared to the control group patients, these differences were not statistically significant. We may hereby deduce the crucial role of the interaction between the embryo HLA profile and maternal KIR genotype in obtaining and maintaining pregnancy.
In the RIF group, we observed a significantly lower rate of biochemical pregnancy in the AA genotype patients compared to those with AB (p=0.0465) and, respectively, BB genotypes (p=0.0423). Other authors found similar frequencies of the KIR haplotypes(14-17). There were authors who noted that activating genotypes were more common in RIF patients than in fertile patients, which is contradictory to our own findings(7,18).
Alexandr et al. published a study performed on patients who underwent double embryo transfers with autologous oocytes, and they observed a higher miscarriage rate in patients with AA KIR haplotype (22.8%) compared to those with BB haplotype (11.1%). At the same time, in patients with double embryo transfers performed with donor oocytes, a smaller live birth rate per IVF cycle was obtained for mothers with AA haplotype (7.5%), compared to those with AB (24.6%) and B haplotype, respectively (21.5%). In patients receiving single embryo transfers with autologous oocyte, there were no statistical differences regarding implantation, miscarriage and live births rates among the three haplotypes(14).
After analyzing each gene, we observed a significantly increased KIR2DS1 activating gene in the control group patients compared to RIF patients (p=0.0372). KIR2DS1 in combination to its specific ligands may generate a powerful activating response on uNK cells(19,20). Some previous studies proved the increased risk of pregnancy complications in the absence of the KIR2DS1 activating gene(21,22), while its presence seems to confer protection against pregnancy adverse events. Wang et al. identified in their study a significantly increased presence of the KIR2DS1 gene in RIF patients compared to healthy patients(23). Other recent researches demonstrated that the activation by HLA-C2 may stimulate the production of soluble factors involved in the migration of primary trophoblast cells and is associated with recurrent miscarriage(22,24,25).
Studies which included, alongside maternal KIR haplotype, the paternal HLA haplotype demonstrated that in the case of AA genotype patients, there was a significantly increased rate of miscarriage when the paternal HLA C2/C2 genotype was associated. Pregnancies with HLA C1/C1 embryos had a 2.5-fold lower rate of miscarriage(22,26).
According to our results, Würfel et al. conducted a study on patients with more than five failed embryo transfers, and they found that the activating KIR2DS1, KIR2DS3 and KIR2DS5 gene types were absent in 78% of cases. In patients where KIR testing proved a deficit of activating genes, the use of granulocyte colony stimulating factor (G-CSF) yielded promising results(27).
The frequency of the KIR3DS1 activating gene was statistically significantly increased in patients who obtained pregnancy compared to those who did not, proving its favorable effect on implantation, but not in the maintaining of pregnancy (there were no statistically significant differences between patients who carried the pregnancy to term and those who miscarried). However, the KIR3DS1 genotype predicts the achievement of pregnancy in a small proportion (r²=0.09).
Our study demonstrated a reduced frequency of the inhibiting KIR2DL5(null) gene in patients who achieved pregnancy compared to those who did not, but this association did not reach statistical significance. At the same time, we obtained a significantly increased frequency in patients who miscarried compared to those who delivered at term. These results led us to believe that the presence of the 2DL5 B(null) gene is more plausibly involved in first-trimester miscarriage than implantation. The results of our study indicate there is a moderate negative correlation between the KIR2DL5-B(null) gene and the evolution of pregnancy (r(34)=0-.46; p<0,01).
Contradictory to our results, Varla-Leftherioti et al. demonstrated a lower frequency of the inhibiting genes KIR2DL1, KIR2DL2 and KIR2DL3, both in the endometrial tissue and the peripheric blood in patients with several miscarriages, compared to fertile patients(28). Sharkey et al., in a study performed in 2015, identified a reduced expression of the KIR2DL1 receptor in women with RIF carriers of the C2 al HLA epitope(22,29). Some studies identified the AA haplotype of KIR2DL1 as being the most powerful inhibitor in the interaction with HLA-C2. This combination between the maternal AA haplotype and the paternal HLA-C2 leads to defective placentation and miscarriage due to a lack of sufficient cytokine secretion required to remodel spiral arteries(5,15,30,31).
The inhibiting KIR3DL1 gene presents the highest polymorphic variation of the entire KIR genes family(32-35). It is incriminated in immune-mediated cardiovascular diseases and, to a lesser extent, in implantation disorders(36). Some studies reported a lower incidence of this gene in patients with more than three miscarriages(37,38). In our study, the KIR3DL1 gene was identified in 100% of the AA and AB haplotypes. We found no association between its expression and pregnancy and abortion rates.
The discrepancies between results obtained by different researchers may have two explanations. The first would be that studies were performed on patient populations with varying geographic backgrounds. Alexandr et al. enrolled European Caucasian patients, Faridi et al. included Northern Indian patients, while Yin et al., Jiang et al. and Wu et al. performed their studies on Chinese women(7,39,40). Secondly, none of the studies took into account the involvement of aneuploidy in the rate of miscarriage, being well established that chromosomopathy is responsible for pregnancy loss in a substantial proportion of cases.
Immune regulation bears a key role in the success of reproduction. The outcomes regarding the clinical relevance of the number of peripheric and uterine cells, as well as their cytotoxic activity offers inconclusive results. Future detailed studies are mandatory to ascertain the predictive value of these cells on the obstetric outcome.
Conclusions
Our study demonstrates that, even though the AA genotype is associated more commonly with implantation failure and with first-trimester abortion, its frequency of occurrence in the group of fertile women is similar to that of patients with RIF. The interaction between the embryo HLA type and maternal KIR profile is essential for the success of IVF and for the obstetric outcome.
The KIR3DS1 gene has a positive influence on implantation, but not on pregnancy maintenance, while the 2DL5B(null) gene is more involved in first-trimester abortion than implantation. The evaluation of KIR genes is a promising diagnostic tool for infertility and for RIF patients as potential treatment targets.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Koot YEM, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012 Dec 1;1822(12):1943–50.
-
Toth B, Würfel W, Germeyer A, Hirv K, Makrigiannakis A, Strowitzki T. Disorders of implantation – are there diagnostic and therapeutic options?
-
J Reprod Immunol. 2011 Jun 1;90(1):117–23.
-
Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014 Jan 1;28(1):14–38.
-
Polanski LT, Baumgarten MN, Quenby S, Brosens J, Campbell BK, Raine-Fenning NJ. What exactly do we mean by ‘recurrent implantation failure’? A systematic review and opinion. Reprod Biomed Online. 2014 Apr 1;28(4):409–23.
-
Flores AC, Marcos CY, Paladino N, Arruvito L, Williams F, Middleton D, et al. KIR receptors and HLA-C in the maintenance of pregnancy. Tissue Antigens. 2007;69(SUPPL. 1):112–3.
-
Moffett A, Chazara O, Colucci F, Johnson MH. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: too early for clinical intervention. Reprod Biomed Online. 2016;33(6):763–9.
-
Faridi RM, Agrawal S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Human Reproduction. 2011;26(2):491–7.
-
Downing J, D’Orsogna L. High-resolution human KIR genotyping. Immunogenetics. 2022;74(4):369–79.
-
Roe D, Williams J, Ivery K, Brouckaert J, Downey N, Locklear C, et al. Efficient Sequencing, Assembly, and Annotation of Human KIR Haplotypes. Front Immunol. 2020;11:582927.
-
Cisneros E, Moraru M, Gómez-Lozano N, Muntasell A, López-Botet M, Vilches C. Haplotype-Based Analysis of KIR-Gene Profiles in a South European Population—Distribution of Standard and Variant Haplotypes, and Identification of Novel Recombinant Structures. Front Immunol. 2020;11:450.
-
Bolarín JM, Pérez-Cárceles MD, Luna A, Minguela A, Muro M, Legaz I. Killer cell immunoglobulin-like receptors (KIR) genes can be an adequate tool in forensic anthropological studies: evaluation in a wide Caucasian Spanish population. Australian Journal of Forensic Sciences. 2021;53(3).
-
Yin C, Hu L, Huang H, Yu Y, Li Z, Ji Q, et al. Genetic polymorphism and evolutionary differentiation of Eastern Chinese Han: a comprehensive and comparative analysis on KIRs. Scientific Reports. 2017;7(1):1–13.
-
Long W, Shi Z, Fan S, Liu L, Lu Y, Guo X, et al. Association of maternal KIR and fetal HLA-C genes with the risk of preeclampsia in the Chinese Han population. Placenta. 2015;36(4):433–7.
-
Alecsandru D, Garrido N, Vicario JL, Barrio A, Aparicio P, Requena A, et al. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Human Reproduction. 2014;29(12):2637–43.
-
Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, et al. Maternal KIR in Combination with Paternal HLA-C2 Regulate Human Birth Weight. The Journal of Immunology. 2014;192(11):5069–73.
-
Alecsandru D, Garcia-Velasco JA. Immune factors in recurrent implantation failure. Recurrent Implantation Failure: Etiologies and Clinical Management. 2018;1:93–102.
-
Djulejic E, Petlichkovski A, Trajkov D, Dimitrov G, Alabakovska S. KIR Gene Frequencies in Women with Infertility Problems. South East European Journal of Immunology. 2015;1(1):1–5.
-
Nowak I, Wilczyńska K, Wilczyński JR, Malinowski A, Radwan P, Radwan M, et al. KIR, LILRB and their Ligands’ Genes as Potential Biomarkers in Recurrent Implantation Failure. Arch Immunol Ther Exp (Warsz). 2017;65(5):391–9.
-
Barry F, Benart L, Robert L, Gala A, Ferrières-Hoa A, Loup V, et al. Interactions HLA-C KIR et anomalies de la placentation: implications dans les issues de grossesses obtenues en AMP. Gynécologie Obstétrique Fertilité & Sénologie. 2022;50(9):600–9.
-
Sivori S, Carlomagno S, Falco M, Romeo E, Moretta L, Moretta A. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood. 2011;117(16):4284–92.
-
Wang S, Li YP, Ding B, Zhao YR, Chen ZJ, Xu CY, et al. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. Journal of Obstetrics and Gynaecology Research. 2014;40(5):1288–95.
-
Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, et al. Maternal uterine NK cell–activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123(10):4264–72.
-
Wang S, Zhao YR, Jiao YL, Wang LC, Li JF, Cui B, et al. Increased activating killer immunoglobulin-like receptor genes and decreased specific HLA-C alleles in couples with recurrent spontaneous abortion. Biochem Biophys Res Commun. 2007;360(3):696–701.
-
Yang X, Meng T. Killer-cell immunoglobulin-like receptor/human leukocyte antigen-C combination and ‘great obstetrical syndromes’ (Review). Exp Ther Med. 2021;22(4):1–10.
-
Dambaeva S v., Lee DH, Sung N, Chen CY, Bao S, Gilman-Sachs A, et al. Recurrent Pregnancy Loss in Women with Killer Cell Immunoglobulin-Like Receptor KIR2DS1 is Associated with an Increased HLA-C2 Allelic Frequency. American Journal of Reproductive Immunology. 2016;75(2):94–103.
-
Alecsandru D, García-Velasco JA. Why natural killer cells are not enough: a further understanding of killer immunoglobulin-like receptor and human leukocyte antigen. Fertil Steril. 2017;107(6):1273–8.
-
Würfel W, Santjohanser C, Hirv K, Bühl M, Meri O, Laubert I, et al. High pregnancy rates with administration of granulocyte colony-stimulating factor in ART-patients with repetitive implantation failure and lacking killer-cell immunglobulin-like receptors. Human Reproduction. 2010;25(8):2151–2.
-
Varla-Leftherioti M, Spyropoulou-Vlachou M, Keramitsoglou T, Papadimitropoulos M, Tsekoura C, Graphou O, et al. Lack of the appropriate natural killer cell inhibitory receptors in women with spontaneous abortion. Hum Immunol. 2005;66(1):65–71.
-
Sharkey AM, Xiong S, Kennedy PR, Gardner L, Farrell LE, Chazara O, et al. Tissue-Specific Education of Decidual NK Cells. The Journal of Immunology. 2015;195(7):3026–32.
-
Yang X, Yang E, Wang WJ, He Q, Jubiz G, Katukurundage D, et al. Decreased HLA-C1 alleles in couples of KIR2DL2 positive women with recurrent pregnancy loss. J Reprod Immunol. 2020;142:103186.
-
Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120(11):4102–10.
-
Ahn RS, Moslehi H, Martin MP, Abad-Santos M, Bowcock AM, Carrington M, et al. Inhibitory KIR3DL1 alleles are associated with psoriasis. British Journal of Dermatology. 2016;174(2):449–51.
-
Saunders PM, Vivian JP, O’Connor GM, Sullivan LC, Pymm P, Rossjohn J, et al. A bird’s eye view of NK cell receptor interactions with their MHC class I ligands. Immunol Rev. 2015;267(1):148–66.
-
Augusto DG, Petzl-Erler ML. KIR and HLA under pressure: evidences of coevolution across worldwide populations. Hum Genet. 2015 Sep 10;134(9):929–40.
-
Dizaji Asl K, Velaei K, Rafat A, Tayefi Nasrabadi H, Movassaghpour AA, Mahdavi M, et al. The role of KIR positive NK cells in diseases and its importance in clinical intervention. Int Immunopharmacol. 2021;92:107361.
-
Ormiston ML, Chang C, Long LL, Soon E, Jones D, Machado R, et al. Impaired natural killer cell phenotype and function in idiopathic and heritable pulmonary arterial hypertension. Circulation. 2012;126(9):1099–109.
-
Akbari S, Shahsavar F, Karami R, Yari F, Anbari K, Ahmadi SAY. Recurrent Spontaneous Abortion (RSA) and Maternal KIR Genes: A Comprehensive Meta-Analysis. JBRA Assist Reprod. 2020;24(2):197.
-
Ataei M, Mirzaei M, Inanloo F, Maleki N, Saee Rad S, Mastooreh Noorbakhsh S, et al. KIR3DL1 gene genotype in patients with spontaneous recurrent abortion. Archivos Venezolanos de Farmacología y Terapéutica. 2021;40(2):120–8.
-
Jiang W, Johnson C, Jayaraman J, Simecek N, Noble J, Moffatt MF, et al. Copy number variation leads to considerable diversity for B but not A haplotypes of the human KIR genes encoding NK cell receptors. Genome Res. 2012;22(10):1845–54.
-
Wu J, Lainer L. Natural killer cells and cancer. Adv Cancer Res [Internet]. 2003 [cited 2022 Sep 24];90:127–35.