Oncofertility is a new branch of medicine, developed from the most frequent long-term side effect of cancer therapy on young women – infertility. Young survivors who have not completed their family yet have now a chance given by oncofertility procedures. The main strategies used in preserving fertility in oncology patients are: ovarian stimulation followed by cryopreservation of oocytes, transposition of the ovaries before radiotherapy, cryopreservation and transplantation of ovarian tissue, and the administration of gonadotropin-releasing hormone (GnRH) agonists, each technique being individualized for each patient.
Oncofertilitatea – o şansă la o viaţă normală pentru tinerele supravieţuitoare ale cancerului
Oncofertility – a chance to a normal life for cancer young survivors
First published: 29 octombrie 2019
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.67.3.2019.2601
Abstract
Rezumat
Oncofertilitatea este o nouă ramură a medicinei, dezvoltată din nevoia de a trata cel mai frecvent efect secundar pe termen lung al tratamentului oncologic – infertilitatea. Tinerele supravieţuitoare care încă nu şi-au completat familia au acum o şansă prin procedurile de oncofertilitate. Principalele tehnici folosite în conservarea fertilităţii pacientelor oncologice sunt: stimularea ovariană urmată de crioprezervarea ovocitelor, transpoziţia ovarelor înainte de iniţierea radioterapiei, crioprezervarea şi transplantarea ulterioară a ţesutului ovarian, administrarea de agonişti GnRH. Alegerea procedurii se face individualizat, pentru fiecare pacientă în parte.
Introduction
Oncofertility is a new branch of medicine, a border between oncology and reproductive medicine that combines different methods of reproductive medicine in order to maximize women’s chances of future fertility.
Nowadays, because of new screening methods, remarkable treatments and early diagnosis, the survival has increased for oncology patients, but with a very high cost – infertility.
Between 1991 and 2012, reports show a 23% drop in cancer mortality. About 5% of women diagnosed with cancer are at reproductive age(1).
Materials and method
We performed a systematic review of published literature describing the effects of cancer treatment on fertility and the current strategies used for preserving fertility in oncology patients, using the PubMed and Medline databases.
Risk of infertility related to chemotherapeutic agent
Aggressive chemotherapy, especially when using an alkylating agent (which has a higher gonadotoxic potential) and radiotherapy, cause premature ovarian failure due to destruction of the ovarian reserve, resulting in infertility, years of hormone replacement therapy and menopause-related symptoms.
Each chemotherapeutic agent has a different mechanism of action and, consequently, a distinct impact on ovary reserve (Table 1).
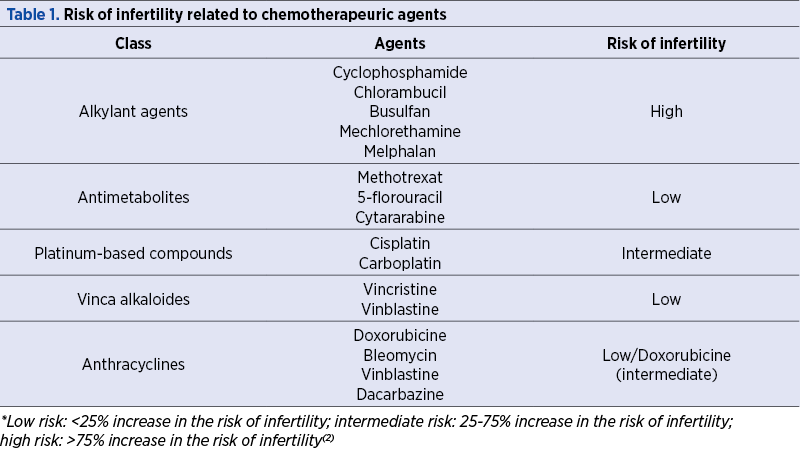
New pharmacological agents with the purpose to protect the ovary during chemotherapy are developing, most of them being still in preliminary stages of study(3).
Women who require gonadotoxic treatment should receive an individual evaluation for fertility preservation. Even nowadays, fertility preservation options are not routinely presented to patients before starting the oncological treatment, and these methods are still underused.
Strategies used in oncofertility
Several strategies are currently available in oncofertility, related to age, type of oncological disease, patients’ profile, probability of gonadal failure, general health at the moment of diagnosis, and the partner’s status.
The current techniques used in preserving fertility in women diagnosed with cancer are: ovarian stimulation followed by cryopreservation of unfertilized oocytes or fertilized oocytes, transposition of the ovaries before radiotherapy, cryopreservation and transplantation of ovarian tissue, and the administration of gonadotropin-releasing hormone (GnRH) agonists during the oncological treatment.
Ovarian stimulation, followed by intracytoplasmic sperm injection (ICSI) and cryopreservation of embryos, is currently the first recommendation for fertility preservation in newly diagnosed cancer patients.
Oocyte banking does not require a partner or a sperm donor at the moment of cryopreservation. With modern techniques as vitrification, more that 60% of mature oocytes survive after thawing(4).
Cryopreservation of ovarian tissue before starting the oncological treatment has recently become one of the most promising techniques for preserving fertility, especially when there is no time for ovarian stimulation. It allows the storage of a large number of primordial and primary follicles. It is the single actual option for preserving fertility in prepubertal oncological patients who do not produce already mature oocytes for freezing(5).
Another great advantage of the method is the posibility to return later in life the ovarian tissue and restore the whole organ function (studies show renewed ovarian endocrine function in 95% of the women) and the posibility to conceive naturally – half of the children born following cryopreservation of ovarian tissue resulted from natural conception(6).
A major worry using this method is the potential risk of grafting malignant cells. The method evaluates the tissue using immunohistochemistry tests and molecular biology techniques, thus the reimplantation of ovarian tissue appeared to be safe, including in patients diagnosed with breast cancer, lymphoma cancers and sarcoma. In leukemia patients, the disease must be in full remission(7).
Also, in patients with BRCA mutations, the ovarian tissue cryopreservation is not an option because it increases the risk of ovarian cancer. For this group of patients, bilateral salpingo-oophorectomy is done for preference after childbearing, so that these patients are candidates for either embryo crypreservation or oocyte cryopreservation(8).
Regarding ovarian transposition, ovaries can be moved from the area receiving radiation; the method is not efficient against chemotherapy effect(9).
Ovarian supression using GnRH agonist during chemotherapy should be considered an option for ovarian preservation in premenopausal patients who are not interested any more in conceiving, but who consider ovarian insufficiency a negative impact on their quality of life. There are no current data available in literature to prove the negative interaction between ovarian supression therapy and chemotherapy. Twelve studies performed between 1966 and 2008 showed that, out of 234 patients who received chemotherapy, 59% of cases had premature ovarian failure (POF) versus 9% after a combination of chemotherapy with a GnRH agonist(10).
Fertility preservation in gynecologic malignancies
Fertility preservation techniques are used in gynecologic malignancies such as cervical cancer, where the focus in preserving fertility consist in conservative surgery (fertility-sparing surgery is a surgical treatment in which one ovary and the uterus are conserved). Patients who are good candidates for fertility sparing surgery don’t receive radiotherapy or chemotherapy(11).
In ovarian malignant pathology, conservative gonadal surgery can be done in the following pathologies: any stage of malignant germ cell tumors, sex cord stromal tumors, stage I invasive epithelial ovarian cancer, and borderline ovarian tumors.
Borderline ovarian tumors appear usually in young women, this is why fertility conservation is an important consideration, and they usually have an excellent survival rate. In borderline ovarian tumors, 15-20% of patients with the serous type will have bilateral involvement; moreover, after conservative surgery, in 10-40% it will reappear(12).
Letrozole during ovarian stimulation should be considered in order to reduce the risk of increasing recurrences. Ovarian cortex cryopreservation is not justified given the possible risks of malignant reseeding; this approach would appear to be contraindicated at least for the moment.
In stage IA of ovarian epithelial cancer, unilateral anexectomy may be done in selected patients who wish to procreate(8). Pregnancy rates in women attempting to conceive after unilateral anexectomy for epithelial ovarian cancer have raised from 27% to 53%(13).
In endometrial cancer, the standard therapeutic manner is hysterectomy with bilateral anexectomy; a fertility sparing method is the continuous administration of progestin (medroxiprogesteron) therapy in very rigorous selection of patients with endometrial hyperplasia or stage IA endometrial adenocarcinoma(14).
Breast malignancy is the leading cause of cancer in women of reproductive age. For many of them, after surgery and chemotherapy it follows five years of con-tinuous treatment with tamoxifen, when they will not be able to attempt pregnancy. Even if tamoxifen is not necessary, current recommendation is to delay pregnancy for at least two years after diagnosis, due to the higher rate of recurrence during this period(15).
Because a high estrogen status is not considered safe for these patients, oocyte retrival can be performed during natural cycles, but usually no more than a single embryo can be obtained. Therefore, because pregnancy rates increase in parallel with the number of embryo transfers, in vitro fertilization stimulation cycles can be done with tamoxifen or letrozole, increasing considerably the number of pregnancies.
Conclusions
The preservation of fertility has become one of the major gain in quality of life for oncology patients undergoing chemotherapy or radiotherapy at reproductive ages and proactively addressing is associated with lower regret and improved quality of life, thus counseling regarding the expected success rates may be difficult in such patients(16).
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
2. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016 Oct; 12(20): 2333–2344.
3. Roness H, Kashi O, Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril. 2016; 105(1):20–29
4. Practice Committee of the American Society for Reproductive Medicine. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013; 99:37–43.
5. Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010; 94:2191–6.
6. Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018 Apr; 35(4):561-570.
7. Gracia CR, Chang J, Kondapalli L, et al. Ovarian tissue cryopreservation for fertility preservation in cancer patients: successful establishment and feasibility of a multidisciplinary collaboration. J Assist Reprod Genet. 2012; 29(6):495–502.
8. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013 Nov; 100(5):1214-23.
9. Terenziani M, Piva L, Meazza C, Gandola L, Cefalo G, Merola M. Oophoropexy: a relevant role in preservation of ovarian function after pelvic irradiation. Fertil Steril. 2009; 91:935.e15–6.
10. Beck-Fruchter R, Weiss A, Shalev E. GnRH agonist therapy as ovarian protectants in female patients undergoing chemotherapy: a review of the clinical data. Hum Reprod Update. 2008; 14:553–561.
11. NCCN Clinical Practice Giudelines in Oncology. Cervical Cancer Version 1. 2017. Available at: https://www.nccn.org/professionals/physiciangls/pdf/cervical.pdf.
12. Morice P. Borderline tumor of the ovary and fertility. Eur J Cancer. 2006; vol. 42, 149-158.
13. Hu J, Zhu LR, Liang ZQ, et al. Clinical outcomes of fertility-sparing treatments in young patients with epithelial ovarian carcinoma. J Zhejiang Univ Sci B. 2011; 12(10):787–795.
14. NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasms, Version 1. 2018. J Natl Compr Canc Netw. 2018 Feb; 16(2):170-199.
15. Vuković P, Kasum M, Raguž J, et al. Fertility preservation in young women with early-stage breast cancer. Acta Clin Croat. 2019; 58(1):147–156.
16. Letourneau JM, Ebbel EE, Katz PP. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012; 118(6):1710–1717.
Articole din ediţiile anterioare
Terapii alternative asociate cu îmbunătăţirea rezultatelor fertilizării in vitro
O problemă importantă cu care se confruntă cuplurile în zilele noastre este infertilitatea, aceasta având impact atât pe plan psihologic şi medical...