The practical advantages of restorative surgery in tubal sterility versus in vitro fertilization (IVF) – especially the avoidance of ovarian stimulation and the risks of multiple pregnancy, the possibility of natural conception and the lower costs – have led the search for new surgical procedures. Performing the tubal isthmic incision as an initial step in solving the tubal pathological processes, both isthmic and ampullary or pavilion, aims to insert the catheter, both in the uterine cavity, facilitating its extraction at the optimum time and along the entire lumen of the restored tube, thus preventing restenosis or tubal obstruction secondary to the intervention. The use of the 0.85 mm catheter, close to the isthmic and interstitial intraluminal tubal anatomical diameter (with ɸ = 0.9 mm), avoids the destruction of the epithelial ciliated cells and non-ciliated endoluminal cells, and enables the postsurgical maintenance of physiological intraluminal diameter of the isthmic incised area. The accuracy of the surgical technique in the case of tubal repermeabilization after tubal ligation ensures a high success rate compared to IVF. In this paper, we approach a staged practical presentation of this pathology in which we highlight the advantages of the described surgical procedure.
Procedeu chirurgical în intervenţiile restauratoare pentru obstrucţiile tubare
Surgical procedure in restoration interventions for tubal obstructions
First published: 12 iulie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.2.2022.6669
Abstract
Rezumat
Avantajele practice ale chirurgiei restauratoare în sterilitatea de origine tubară versus fertilizarea in vitro (FIV) – şi anume, evitarea stimulării ovariene şi a riscurilor de sarcină multiplă, posibilitatea concepţiei naturale şi costuri mai reduse – au impulsionat căutarea unor procedee chirurgicale noi. Efectuarea inciziei istmice tubare ca etapă iniţială în rezolvarea proceselor patologice tubare, atât istmice, cât şi ampulare sau pavilionare, are drept scop introducerea cateterului atât în cavitatea uterină, facilitând extragerea lui la momentul optim, cât şi de-a lungul întregului lumen al salpingei restaurate, astfel împiedicând restenozarea sau obstrucţia tubară secundare intervenţiei. Utilizarea cateterului cu ɸ=0,85 mm, apropiat de diametrul anatomic tubar intraluminal istmic şi interstiţial (cu ɸ=0,9 mm), evită distrugerea prin presiune a celulelor epiteliale ciliate şi nonciliate endotubare ale zonei respective, dar permite şi menţinerea postoperatorie şi în zona incizată istmică a diametrului intraluminal fiziologic. Acurateţea tehnicii chirurgicale în cazul repermeabilizării tubare după ligatura tubară asigură o rată de succes mare în raport cu FIV. În această lucrare, abordăm o prezentare practică etapizată a acestei patologii în care vom sublinia avantajele procedeului chirurgical descris.
Introduction
Surgical plastic and restorative methods on the fallopian tube are based on the anatomical and mechanical principles of reconstitution of the tubal lumen, but they are not solving the deficiency of functional physiological factors, kinetic and secretory functions, epithelial and ciliary, which interferes with the complexity of fertilization.
The results obtained are modest, being related both to the technical skills of the operating team, the quality of the surgical instruments and the materials used, but also to the indications of the surgery. The surgical practice validated two aspects: not every tubal obstruction is suitable for an operation, and the increased frequency of postoperative tubal obstruction.
The operative indication is decided after the sum of investigations which have the purpose to establish the permanent anatomical character and the etiology of the obstruction, the presence of some factors associated in the sterility of the couple, such as sperm quality or sperm ascent research etc. Hysterosalpingography (HSG), laparoscopy and hysteroscopy play a key role in assessing if surgery is appropriate.
The rehabilitation of these surgical techniques is motivated and imposed both by the prohibitive nature of the alternative – in vitro fertilization (IVF) for people with limited financial possibilities, and by sociomedical implications: increased frequency of tubal obstructions, proportional to the number of abortions, high risk of ectopic pregnancies, low rate of favorable results.
Therefore, the absolute indication of surgery is imposed by two factors: the permanent nature of the tubal obstruction, and the cause of infertility.
The contraindications of these categories of interventions imply the existence of a confirmed genital tuberculosis, respectively the dysfunction of the fallopian tube: laparoscopic examination, short, thick and completely sclerotic fallopian tubes. Acute genital inflammation requires a delay of at least six months to perform these tubal restorative procedures(1).
Prior to starting the treatment, cervical cultures are performed for microbial cultures, including Chlamydia, Mycoplasma and Ureaplasma.
Materials and method
Obstructions located in the isthmic or isthmus-interstitial portion of the fallopian tube give the highest percentage of failures in classical repair procedures of tubo-uterine reimplantation.
This finding justified an increased focus on improving surgical techniques, moving from classical repairing surgery to restorative surgery, including microsurgery of the affected fallopian tube.
In the current gynecological surgery treatment, the term “proximal tubal occlusion” describes an obstruction that appears proximal to the fimbria and can develop in the ostium, isthmus or tubal ampulla(3).
Following the diagnosis of proximal tubal occlusion by hysterosalpingography, current studies are considering a selective salpingography, with the fixation of a catheter at the level of the tubal ostium and the application of significant hydrostatic pressure on the tube, a procedure that would help remove the spasm of the tube or remove mucus plugs or detritus. The absence of repermeabilization of the fallopian tube with the persistence of the proximal tubal obstruction requires the transition to the next stage, the surgical one.
The surgical stage involves the option of either hysteroscopic cannulation or tubal segmental resection with anastomosis(3).
Hysteroscopic cannulation with concomitant laparoscopy can repermeabilize isolated and short fibrous segments(3). The tubular cannulation techniques, described before, require specific equipment and experience in the field.
A longer scarred segment or luminal obliteration cannot be corrected by tubular cannulation, requiring segmental surgical resection with anastomosis or IVF(3). From the patient’s point of view, segmental surgical resection with tubal anastomosis has advantages over IVF: avoidance of ovarian stimulation and the risks of multiple pregnancy, and the possibility of natural conception(3). From the perspective of the reasons mentioned before, in this paper our aim is to present our experience in the field.
The old decalogue of the conditions for performing the surgeries remains valid completed with the use of a 20 G catheter from the set for epidural anesthesia with ɸext. = 0.85 mm, fine atraumatic needles with slowly resorbable sutures of 6/0 or 4/0 for sutures, optional 7/0 slow sutures, with microsurgical instruments and materials, electrosurgery electrode, hysterosalpingography device, and methylene blue solution.
From the sample of interventions, we present a case where HSG diagnosed a proximal obstruction of the fallopian tube in the interstitial or isthmic segment. We followed the protocol applied in determining the level of obstruction of the fallopian tube.
To remove a possible corneal uterine polyp in the obstructed tube, we visualized hysteroscopically the integrity of the tubal ostium. The visualization of a cornual uterine polyp requires its hysteroscopic removal, or in the absence of the hysteroscope, by curettage of the uterine horn and subsequent repetition of HSG. Imaging of the obstacle confirms the tubal isthmus-interstitial obstruction.
However, the inclusion of chronic salpingitis as an etiology of tubal isthmus-interstitial obstruction suggests, however, the therapeutic superiority of endometrial curettage, even in the absence of clinical signs of chronic endometritis – bad smelling menstruation, brown pre- or post-menstrual secretions.
We reiterate the need to perform microbial cultures of the cervix, both before small surgeries – HSG, endometrial curettage or hysteroscopy, and intraoperatively on the surface of the tubes and from the Douglas peritoneal pouch.
We also recommend completing the therapeutic algorithm, respectively performing HSG and the curettage of the uterine horns preoperatively, in the interventions of repermeabilization of the fallopian tubes secondary to the tubal ligation procedures as well. Cornual endometrial polyps are targeted in this case.
A laparoscopy performed before surgery provides us the anatomical data about the condition of the fallopian tube, distal to the existing obstacle.
The timing of the procedure is optimal in the first week after the end of menstruation, the anesthesia being the general one with orotracheal intubation (OTI), justified by the possibility of prolonging the operation.
With the improvement of the surgical technique, in agreement with the anesthetist, the spinal anesthesia enhanced with intravenous medication was chosen due to the known advantages: simplicity of execution, reduction of perianesthetic stress, maintaining intraoperative verbal contact with the patient and, last but not least, maintaining muscle tone while maintaining anatomical landmarks. From an anesthetic point of view, the surgeries were performed without any special events, except for hypotension.
In most cases, Heavy Marcaine® was chosen in combination with fentanyl and Dormicum®, with excellent anesthetic results both intraoperatively and postoperatively for up to 1-2 hours.
The classical authors(1) started corticosteroid therapy before the surgery to prevent the adhesions, a treatment used by us in the first interventions (dexamethasone). Through the laparoscopic examination performed 14-21 days postoperatively, we found undesirable reactions: an accentuated adhesion syndrome between the tubal suture line and the omentum, the parietal peritoneum or the uterine posterior wall, which is why we gave up this adjuvant treatment. We replaced dexamethasone with sterile hyaluronic acid gel (Hyalobarrier Gel®) applied topically at the end of the surgery on the uterus and adnexa, the result evaluated laparoscopically at 14-21 days postoperatively being clearly favorable.
The patient’s position is supine, initially with the pelvic limbs flexed to secure the HSG device, then the patient’s lower left limb is horizontalized so that Operator 1 has easy intrapelvic access and the operating table support for the patient’s right lower limb is tilted and fixed so that this pelvic limb is removed and slightly bent, allowing access to the HSG equipment during the operation to perform the ascending instillation with 50 ml of diluted methylene blue solution.
After Pfannenstiel laparotomy, preparation and isolation of the pelvis by lifting the intestinal loops, covered with soft gauze fields soaked in warm saline and examining the lesions, microbial cultures are performed from the bottom of the Douglas peritoneal sac and from the surfaces of the fallopian tubes. Then we put a wire of Bicril® in the “X” median on the anterior face and bottom of the uterus, loosen the adhesions with the coagulation electrode and put a wire of Bicril® in “X” on the lower pole of the ovary, the two wires helping to expose the obstructed tube.
A first instillation is performed with methylene blue which confirms the location of the tubal obstruction at the isthmic or interstitial level.
The first stage of the surgical procedure consists in performing an oblique transverse incision, complete or incomplete, at the level of the tubal isthmus with the upper extremity bended towards the uterine horn (Figure 1 – position 1; 2) at a distance of 5-10 mm from it. The oblique incision increases the approach surface of the obstructed proximal tubal segment and facilitates the insertion of the catheter into the uterine cavity. This is the most difficult maneuver of the intervention, aiming to unclog and maintain the permeability of the tubal canal. If we suspect that the obstacle is interstitial, the distance will be 5 mm, to facilitate its removal. We recommend that the tubal incision should be made with a fine straight scissors, and with the cauterization electrode in order to obtain the perfect hemostasis of the nutritive arteries of the fallopian tube.
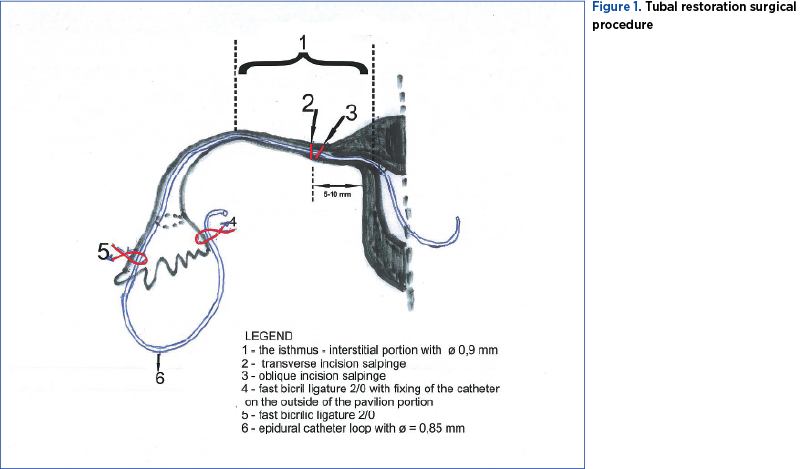
The incomplete oblique incision is correctly executed if we visualize the entire surface of the internal isthmic canal. This oblique isthmic tubal incision respects the vascularization of the internal tubal artery, as well as the extensive adrenergic innervation of the tubal branch of the lateral nerve of the uterus. We recommend this surgical procedure, although recent studies in animals (rabbits) have shown that the surgical denervation of the fallopian tube does not destroy the transport of eggs(2). The salpinx seems to adapt easily to anatomical changes and restrictions(4).
After hemostasis, the ascending instillation with methylene blue is repeated. In the case of the permeability of the internal isthmic duct, we visualize on the surface of the oblique tubal incision a blue dot with ɸ of approximately 0.9 mm. Otherwise, the isthmic epithelial duct has a white color.
In the absence of the permeability of the internal isthmic tubal duct, in a first stage, its unclogging is attempted with the help of the proximal end of the catheter from the epidural kit with ɸ = 0.85 mm. To insert the catheter into the internal tubal canal, having close diameters, we can use two maneuvers, either manual compression of the proximal tubal segment or fixing its serosa with Pèan forceps. We describe in detail the two maneuvers:
-
The manual anteroposterior compression performed lightly determines the protrusion of the internal canal with approximately 1 mm at the surface of the tubal incision, facilitating the insertion of the catheter, maneuver performed by the Operator 1.
-
Operator 2 fixes in the anatomical axis the proximal tubal end with the help of 2-3 fine Pèan forceps, applied on the serosa, and Operator 1 tries to insert the proximal end of the 20 G catheter from the epidural anesthesia set, through the isthmic epithelial duct until it enters the uterine cavity. To facilitate the insertion of the initial end of the catheter through the proximal tubal internal isthmic duct, in addition to the maneuver described above, we recommend the use of the “successive movements” technique, Operator 1, using two fine Pèan forceps, with a forceps insert 0.5-1 cm uterine, with the other forceps Pèan maintains the new position of the catheter, which tends to retract.
If the attempt to insert the catheter into the uterine cavity fails, the ascending instillation is repeated. If the test remains negative, the oblique parallel slices 2 mm thick are sectioned from the isthmus-interstitial portion, successively until we visualize the normal passage of the methylene blue solution. If the normal passage of the methylene blue solution is found, but it is difficult to insert the epidural catheter (ɸ = 0.85 mm) into the uterus, through the interstitial portion, a green i.v. catheter can be used as a guardian (18 G, 1.3 x 45 mm). Through its lumen, the epidural catheter is inserted into the uterus, followed by the extraction of the i.v. catheter through the distal extremity.
The epidural catheter must penetrate the uterine cavity at least 6 cm to form a loop, similar to the Shirodkar procedure(1).
Once in the uterine cavity, the catheter loses the tendency to retract, but it will have to be fixed to the serosa of the proximal tubal portion with a tight suture of Bicril® 4/0. By this fixation we prevent a possible withdrawal of the catheter from the uterine cavity when manipulating the distal end.
After inserting the catheter into the uterine cavity, we move on to the second stage of the operation, that of visualizing the permeability and quality of the internal duct of the distal portion of the fallopian tube (isthmus-ampullary-pavilion) by diluted methylene, applied to the orifice of the internal pavilion duct, kept tight on the tip of the syringe. If the test is negative, in the first stage we perform the unclogging of the isthmic duct with the help of the distal end of the catheter.
If the test remains negative, according to the Erhler procedure(1), it is cut by successive isthmic cross-sections, slice by slice, until the descending test with colored solution shows a good permeability.
After shortening the free end of the epidural catheter to about 20 cm, the end is inserted in the same way as above, by successive movements, through the distal isthmic tubal duct, crossing the isthmus-ampullary junction, the tubular ampulla and overcoming the salpinx fimbriae by 7-8 cm. If the maneuver is difficult (altered anatomy of chronic tubal infections), we recommend serial cross-sections of the stenotic tubal segments, with catheterization of the entire lumen and associated surgical gestures (hemostasis, parietal suture).
After the endotubal catheter is stretched, the Bicril® 4/0 thread that secured the catheter to the serosa of the proximal tubal portion is sectioned and the surgical procedure is completed by suturing the tubal incisions on the catheter.
In case the gynecology service is equipped with instruments and microsurgical materials and the corresponding lenses, we use 7/0 slow resorbable threads, the suturing of the tubal muscles being performed with separate threads in each quadrant. The closure of the tubular serosa requires slowly resorbable threads 6/0 (polydioxanone), the suture being separated or continuous.
The accidental or intentional mesosalpinx incision, in the latter case to facilitate tubal maneuvers, is sutured with 6/0(3) or 4/0 slowly resorbable separate threads.
In the absence of instruments and microsurgical materials, the tubular suture on the catheter can also be performed by applying four separated slowly resorbable 4/0 serum-muscular threads, one in each quadrant.
The major importance of the surgical procedure described before, namely the isthmic incision with the insertion of one end of the catheter into the uterine cavity, lies in the fact that it can be applied as an initial step in solving obstacles or pathological processes located in the middle or distal portion of the fallopian tube, with the advantage of preventing clogging and, after healing of the suture, with easy removal of the catheter. The opportunity of this procedure is given by the existence of a minimum chance of direct insertion of the epidural catheter from the tubular ampulla or the pavilion through the internal isthmic canal to the uterine cavity. We insist that the isthmic tubal suture should be performed as correctly as possible so as not to later become an impediment in the process of obtaining uterine pregnancy.
If we intraoperatively find an inflammatory pathological sinuosity of the fallopian tube, persistent even after lysis of the mesosalpinx adhesions, where there is the passage of the dye and no catheter permission, to reduce the subsequent risk of tubal pregnancy, we consider a transverse incision needed at the level of the sinuous process, hemostasis with the electrocautery, the extraction of the catheter from the proximal internal duct and its reintroduction into the distal internal duct of the fallopian tube in order to exceed the level of the pavilion fimbria. The suture of the tubal incision is made in the manner described above. This procedure restores the curvature of the salpinx to its natural anatomy.
The pavilionar end of the catheter is sectioned 5-6 cm away from the pavilion fimbriae, leaving either free or fixed to the nearest avascular area of the serous broad ligament, respectively with a 2/0 rapid resorption Bicril® thread with fast absorption. In the case of terminal neosalpingostomy, in order to maintain a sufficiently functional pavilion opening, the intraperitoneal extremity of the inserted catheter is fixed under the pavilion with Bicril® rapid 2/0 (Figure 1 – position 4), on the outside of the pavilion portion. When it is decided to remove the catheter, if the fixation wire has not resorbed, a gentle traction will favor the formation of a small and nonbleeding serous scar. We recommend this serous fixation of the pavilion end of the catheter because we had a case where the catheter was removed through the endocolus four days postoperatively, and the respective tubal extremity of the pavilion was obstructed – hydrosalpinx seen in ultrasound.
After cleaning the peritoneal cavity, the sterile hyaluronic acid gel is applied, in a layer of 1-2 mm on all surfaces of the internal genitals (ovaries, fallopian tubes, uterus), which prevents the formation of adhesions in the critical period, the first postoperative week. Washing the surfaces of the internal genitals with warm saline to avoid attaching the protective gel to the target organs should be avoided. It is also necessary to perform a thorough hemostasis with the electrocautery, because the application of the gel prevents the obliteration of small mass hemorrhages by the mechanical barrier of the omentum.
In the case of tubal obstructions of an inflammatory nature, contrary to classical opinions, we recommend leaving a drain in the Douglas pouch, the hyaluronic acid preventing the multiplication of adhesion process.
The extraction of the isolation fields and the restoration of the abdominal wall in anatomical layers complete the surgery. Antibiotic therapy, analgesics, postoperative nadroparins and early mobilization.
The removal of the uterine end of the catheter is easy, taking a few minutes, and it is performed after the next menstruation, by dilating the cervical canal under i.v. general anesthesia. Incidentally, the remaining catheter segment may retract intraperitoneally. Being made of biologically inert material and located in a buffer zone with sterile gel (Hyalobarrier Gel®), the risk of foreign body reaction is absent. The aforementioned incident (intraperitoneal withdrawal of the catheter) can be prevented by providing a second fixation point – suture with Bicril® fast 2/0 of the catheter loop at the upper end of the tubal pavilion (Figure 1 – position 5). The solution proposed by us was confirmed by the surgical practice in all cases in which it was used.
The quality of the permeabilization is verified by HSG 1-2 months postoperatively.
In the case of restoring tubal permeability after bilateral tubal ligation, the existence of anatomically healthy salpinges allowed us to initiate tubal clearance from the distal extremity (tubal pavilion) to the proximal one, using the techniques outlined before.
Discussion and results
This surgical technique for resolving tubal obstructions can be performed in any gynecology department because it does not require sophisticated instruments or equipment and it does not require microsurgery.
The experience of the gynecologist is gained not only through the accumulated theory, but especially through the surgical practice.
The surgical technique in question is recommended in interstitial or isthmic tubal obstructions, but it can be imposed, as an initial stage, in obstructions of the ampullary or pavilion segment of the fallopian tube.
The classic interventions, half a century ago, of tubo-uterine reimplantation, either at the level of the uterine horn (Palmer) or through the posterior wall of the uterus (Bourg), bring important anatomical changes with the following consequences:
1. Significant shortening of the fallopian tube, which exposes to the rapid migration of the egg. Current studies indicate that egg nesting depends on synchronization between endometrial and egg development(5,6).
The fallopian tube provides an important action to maintain the egg, to allow the endometrium to become receptive and the blastocyst to become capable of implantation, respectively a period of approximately 80 hours of which 90% is in the ampoule(2). Therefore, the restored horn must be at least 5 cm long.
2. Unwanted passage of menstrual blood into the peritoneum due to uterine-tubal incontinence, with the risk of dysmenorrhea or even endometriosis(1).
3. In reimplants performed at the level of the uterine horn (Palmer), there is a risk of dehiscence of the myometrium during pregnancy, with incomplete subperitoneal ruptures(1).
4. The classical techniques of tubo-uterine reimplantation (in our personal experience, we used only the Palmer procedure) we do not consider them anatomical, the argument being that the two ears (5 mm each) can rarely be fixed continuously with the uterine mucosa, creating distances and thus the sperm has a sinuous path to enter the tubal lumen.
5. In the Erhler procedure (1963) silastic prostheses with ɸ = 0.4 mm had been used, which easily penetrated through the tubal lumen, but which could have favored a restenosis of the tubal lumen up to ɸ = 0.4 mm at the level of the anastomoses, with the difficult transport and the possibility of increasing the frequency of tubal loads.
Approximately 50% of the length of the tubal lumen (the interstitial and isthmic portion) has an internal diameter of approximately 0.9 mm (Figure 1 – position 1).
Compared to the polyethylene tutor with ɸ = 1.2-1.7 mm used in the classic surgery of tubo-uterine implantation (Sârbu procedure), in the presented procedure we used the epidural catheter with ɸ = 0.85 mm, to maintain the entire length of the tubal lumen a caliber as close as possible to the physiological one and without creating compression on the cells of the endotubal epithelium.
Like the Erhler procedure(1), our procedure does not shorten the fallopian tube, and through the catheter used we prevent restenosis, while avoiding tubal incontinence and the rapid passage of the egg.
Also, the permissive flexibility of the proximal end of the catheter does not produce false paths, a risk assumed when using the buttoned stylus, and molds to the isthmus-interstitial curvature of the fallopian tube, making it permeable and preventing reobstruction.
In the case of pathological processes located on the middle or distal segment of the fallopian tube, where the luminal diameter of the hydrosalpinx does not exceed 3 cm – indication by IVF), end-to-end anastomosis, medioampular salpingostomy plastic and repairing surgery (Polloson, Holden-Sovak) or end-lateral neosalpingostomies (Polloson) – due to irreversible changes in the tubal walls and postoperative adhesions, lead to a high percentage of reobstructions (43-59% Palmer)(1). For this reason, we consider it beneficial in these interventions, after the specific restoration of the pathological tubal segment, to use the isthmic oblique incision 5-10 mm from the uterine horn, for implantation of the catheter in the uterine cavity for extraction, as well as along the entire length of the tubal lumen, preventing thus reobstruction.
Also, the exposed surgical procedure may be indicated in the tubal sterility, with hysterosalpingographical permeable fallopian tubes, but with sinuous trajectory, coiled and elongated, where the lysis of the mesosalpinx adhesions and the implantation of the intratubal catheter bring the fallopian tube back to physiological anatomy. This indication is feasible with the following amendment: after one year of ovulation monitoring, antibiotic treatments according to the antibiogram and assessing the associated factors such as sperm quality, sperm rise etc., it is established that the only cause of infertility is the pathological anatomy of the fallopian tube.
To avoid postoperative adhesion, we use sterile hyaluronic acid gel, as described before.
The results of plastic and restorative interventions on the fallopian tube are not legitimized by the percentage of repermeabilization cases, but by the number of uterine pregnancies obtained, noting that these operations do not solve the deficiency of kinetic and secretory functional factors.
Today, the number of sterile plastic and restorative microsurgery clinics in Romania is insufficient to cover the need for specialized services.
Until this situation is solved, our procedure, not necessarily requiring microsurgery gestures, can be applied in other gynecology services with addressability in this field.
Our experience, based so far on a statistically insignificant number of cases, does not allow us to make an objective assessment of the success rate of surgery proposed in the treatment of tubal sterility.
Conclusions
Although they require high costs related to equipment, instruments and medication, in vitro fertilization procedures bring favorable results in one in four cases treated.
On the other hand, we believe that plastic and tubal restorative interventions, with precise indications, should be reintroduced as an intermediate stage in the complex treatment of sterility, an observation also supported by the fact that, before performing the IVF procedure, surgical resolution of hydrosalpinx is appropriate.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Sîrbu P. Chirurgie ginecologică, vol. I, Ed. Medicală, Bucureşti, 1981.
-
Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility, 8th Edition, 2010.
-
Hoffman B, Schorge J, Schaffer J, et al. Williams Gynecology, 2nd Edition, Chapter “Treatment of infertile couples”, pp. 529-553, 2012.
-
Molloy D, Deambrosis W, Keeping D, Hynes J, Harrison K, Hennessey J. Multiple-sited (heterotopic) pregnancy after in vitro fertilization and gamete intrafallopian transfer. Fertil Steril. 1990;53(6):1068-1071.
-
Shoham Z, Zosmer A, Insler V. Early miscarriage and fetal malformations after induction of ovulation (by clomiphene citrate and/or human menotropins), in vitro fertilization, and gamete intrafallopian transfer. Fertil Steril. 1991;55(1):1-11.
-
Olivennes F, Kerbrat V, Rufat P, et al. Suivi d’une cohorte de 422 enfants agés de 6 a 13 ans et conçus en fécondation in vitro [Follow-up of a cohort of 422 children aged from 6 to 13 and conceived by in vitro fertilization]. Contracept Fertil Sex. 1997;25(5):XIII-XVIII.