Osteopenia of prematurity (OP) – also called metabolic bone disease (MBD) or rickets of prematurity – is a late complication of preterm birth, consisting of decreased bone mineralization in patients with low gestational age and birth weight. The disease can be expressed in various degrees of severity, mostly mild. The real incidence of the disease is not known due to the lack of international consensus regarding its definition, but it is approximated at 50% of babies under 1000 g. Despite the actual knowledge about its physiopathology and advances in the nutritional strategies for very-low-birth-weight (VLBW) infants, osteopenia of prematurity is still present, probably underestimated. Most of the times, no specific signs or symptoms are identified. If present, these are represented by growth and length deficits, signs of classical rickets, respiratory distress syndrome, or difficulty to wean from ventilatory support, due to impaired thoracic compliance. The risk factors for an increased gravity of OP are both prenatal and postnatal, and the genetic predisposition could be involved. We report the case of a 24-week gestation ELBW baby with a complicated clinical course, involving respiratory, neurologic, cardiovascular and digestive alterations, who was diagnosed at 5 months of age with a severe osteopenia of prematurity expressed as multiple subsequent fractures which raised problems of differential diagnosis and management. The retrospective case analysis showed that a combined effect of prenatal and postnatal risk factors (extreme prematurity, maternal chorioamnionitis, prolonged TPN, use of corticotherapy, methylxanthines, diuretics, immobilization and sedation) have conducted to the extreme severity of the disease in our patient. This article intends to emphasize the importance of surveillance of the nutritional status of both mother and infant. The correct pregnancy monitoring and promoting an aggressive nutritional strategy for extremely-low-birth-weight infants could prevent an aggressive course of the disease and poor long-term outcomes.
Un caz sever de osteopenie de prematuritate
A severe case of osteopenia of prematurity
First published: 24 mai 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ObsGin.70.1.2022.6516
Abstract
Rezumat
Osteopenia de prematuritate – denumită şi boala metabolică osoasă sau rahitismul prematurului – este o complicaţie tardivă după naşterea prematură, caracterizată prin scăderea mineralizării osoase la nou-născuţii cu greutate şi vârstă gestaţională mică la naştere. Boala se poate manifesta cu grade diferite de severitate, majoritatea în forme uşoare. Deoarece nu există un consens internaţional în ceea ce priveşte definiţia bolii, incidenţa reală a acesteia nu este cunoscută, dar se estimează că afectează aproximativ 50% din prematurii cu greutatea sub 1000 g. În pofida cunoştinţelor actuale despre fiziopatologia bolii şi a progreselor în ceea ce priveşte strategia nutriţională a prematurilor cu greutate extrem de mică la naştere, osteopenia de prematuritate este încă prezentă, fiind probabil subestimată. În cele mai multe cazuri, nu se evidenţiază semne sau simptome specifice. Dacă sunt prezente, ele se exprimă ca deficit de creştere în greutate şi lungime, semne clasice de rahitism, sindrom de detresă respiratorie sau dificultate de înţărcare de pe ventilator, din cauza afectării complianţei toracice. Factorii de risc pentru gravitatea bolii se regăsesc atât prenatal, cât şi postnatal, fiind demonstrată şi o predispoziţie genetică. Prezentăm cazul unui nou-născut prematur de 24 de săptămâni de gestaţie, cu greutate extrem de mică la naştere şi cu o evoluţie clinică complicată cu afectare respiratorie, neurologică, cardiovasculară şi digestivă, diagnosticat la vârsta de 5 luni cu o formă severă de osteopenie de prematuritate, manifestată prin multiple fracturi concomitente, care au ridicat probleme de diagnostic diferenţial şi de conduită terapeutică. Analiza retrospectivă a cazului a evidenţiat prezenţa factorilor de risc pre- şi postnatali: prematuritatea extremă, corioamniotită maternă, nutriţie parenterală totală prelungită, utilizarea corticoterapiei, a metilxantinelor şi diureticelor, imobilizarea şi sedarea, efectul cumulat al acestora conducând la evoluţia dramatică a bolii. Articolul doreşte să sublinieze importanţa supravegherii statusului nutriţional al gravidei şi nou-născutului, a consulturilor periodice în sarcină şi a introducerii în practica standard a unei strategii nutriţionale agresive pentru nou-născutul cu greutate foarte mică, concomitent cu monitorizarea biochimică, pentru a preveni evoluţia gravă a bolii şi prognosticul nefavorabil al acesteia pe termen lung.
Introduction
Osteopenia of prematurity (OP) – also called metabolic bone disease or rickets of prematurity – represents a decrease in bone mineralization or a reduced bone mass content (BMC) due to insufficient pre- and postnatal nutrients intake; it represents a late complication after preterm birth and it can be expressed in different clinical forms, with various degrees of severity.
The real incidence of the disease is not known due to the lack of international consensus regarding its definition, but OP affects especially newborns with birth weight (BW) below 1500 g. The incidence is higher at smaller gestational ages (GA) and low birth weight (BW): 23-32% of newborns under 1500 g and 50% of newborns under 1000 g develop this complication(1). Despite advances in the nutritional strategies for very-low-birth-weight (VLBW) and extremely-low-birth-weight (ELBW) infants, osteopenia of prematurity is still present, probably underestimated, as diagnostic tools for newborns are limited.
Most of the times, OP has no signs/symptoms, being diagnosed through specific tests where screening programs are implemented. If the signs are present, they appear usually at 4-12 weeks of age as growth or length deficits (head circumference is usually spared) and signs of classical rickets: hypotonia, frontal bossing, enlarged fontanel, craniotabes, rachitic rosary, Harrison costal grooves, and deformities of the joints (wrists, knees, ankles). Nevertheless, patients with multiple risk factors can experience fractures of pathological bones.
We report the case of a ELBW premature baby with severe OP and comorbidities which led to a dramatic clinical picture with multiple fractures.
Case report
The patient M.G. was born at 24 weeks of gestation with a body weight of 800 g, from a pregnancy with associated infectious risk (rupture of membranes four weeks before delivery, chorioamnionitis, inflammatory syndrome), being diagnosed at birth with:
-
respiratory distress syndrome (RDS) requiring respiratory support
-
early-onset neonatal sepsis
-
intraventricular hemorrhage (stage III)
-
patent ductus arteriosus (PDA).
During hospitalization, the baby developed multiple complications: chronic lung disease (CLD), necrotizing enterocolitis (NEC), acute renal failure (ARF), stage III retinopathy of prematurity (ROP) with plus disease, anemia, cardiac hypertrophy. Therefore, the baby was on prolonged cardiorespiratory support and, despite different ventilatory strategies and medications, weaning was difficult, with repeated relapses of respiratory distress syndrome. Although enteral nutrition was tempted early, the baby did not tolerate oral feeding, with abdominal distension and gastric residuals, so total parenteral nutrition (TPN) was necessary for 39 days of life, with flat weight curve. Increases in nutritional intake were followed by hyperglycemia, high BUN (blood urea nitrogen) and hyperlipemia, and fluid administration had to be limited to promote PDA closure. TPN solutions contained glucose, proteins, lipids, vitamins and calcium gluconate 10% 2 mEq/kg/day from birth; no supplement of parenteral phosphate was available.
Once enteral feeding was tolerated, the baby was fed orogastric with expressed mother milk, with slow progression in the amounts per meal. Milk fortifiers were added at 60 days of life, when the total volume of milk was approximately 100 ml/day. Vitamin D (Baby Guard DHA® 400 UI/day) was added once enteral feeding was tolerated.
Weight gain has started in the 23rd day of life and remained suboptimal for approximately eight weeks. The baby experienced a period of extrauterine growth restriction. Respiratory condition progressed to chronic lung disease requiring treatment with methylxanthine, diuretic and corticosteroids, and intermittent sedation during mechanical ventilation or as treatment for seizures episodes.
For advanced stage of retinopathy of prematurity, the baby received intraocular injection with vascular endothelial growth factor (VEGF) inhibitors.
At 5 months of life, the patient was still receiving oxygen (free flow, FiO2 30%), had mild respiratory effort, was fed exclusively enteral with expressed mother milk with added fortifier both through orogastric tube and by bottle, with a satisfactory weight gain up to 3000 g. The clinical examination at that point also revealed the presence of umbilical and bilateral voluminous inguinal hernia, firm but reducible. In this context, sudden crying spells and episodes of extreme agitation were noted, considered initially to be related to colics/gastroesofageal reflux and/or abdominal wall pathology. Care has been given to address these problems, with no significant alleviation of the symptoms.
The subsequent clinical examination revealed slight and partial alteration of the mobility of the right upper limb, without signs of local inflammation (no tumor, rubor or calor).
The laboratory tests showed no markers for infection or inflammation. The radiologic examination showed a fracture in the upper third of the right humerus and a consolidated fracture of the left humerus (callus), although no traumatic event had been noted (Figure 1).
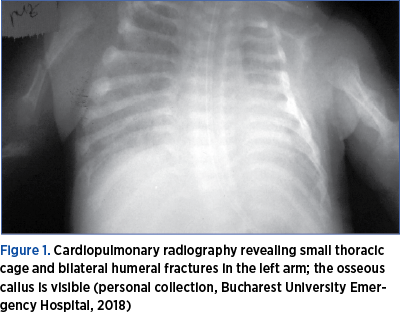
These findings raised problems of differential diagnosis, especially with an osteomyelitis within a late onset sepsis, which was discarded after orthopedic referral, imagistic exams and laboratory test results: two negative blood cultures, negative CRP, procalcitonin below 2 mg/dL.
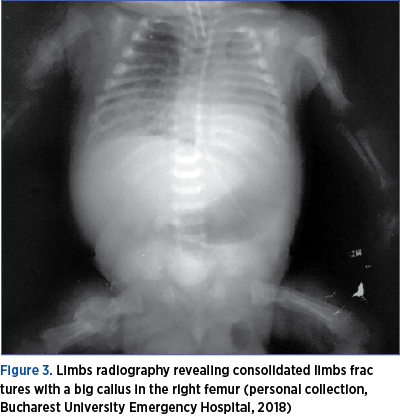
Although care was given to reduce any further risk, in the next two weeks, X-ray examinations revealed other two successive fractures (left and right femur), although no traumatic events/maneuvers were recorded, the baby receiving only standard care (Figures 2 and 3).
The laboratory tests revealed: normal values for total and ionized calcium and magnesium, normal values for vitamin D3 and parathormone (PTH), but abnormal low values for phosphate and high alkaline phosphatase level (ALPl): 2.6 mg/dL and, respectively, 640 UI/L.
The therapy consisted of: limbs immobilization, minimal handling, increased nutritional supplements (protein, P, Ca, Mn, Zn), increased vitamin D dose (1200 UI/zi), analgesics (Perfalgan® 10 mg/kg/dose every 6 hours); in order to minimize the secondary osseous effects of other medications, the treatment with steroids and miofilin, which was given for chronic lung disease (CLD), was discontinued, and the diuretic treatment meant to decrease cardiac overload was adjusted (furosemide every other day instead of daily). The balance between high nutritional and caloric intake and adequate diuresis/excretion is of huge importance and difficult to find for a patient with extrauterine growth restriction, CLD and signs of cardiac failure.
During the aggressive supplementation, we had to monitor strictly the serum level of phosphate, calcium (total/ionized), ALP, glucose, BUN, lipids and electrolytes.
With these interventions, the clinical course of the disease went towards fractures consolidation (2-3 weeks), although bone mineralization remained deficitary. An improvement of the biochemical markers values was noted. Unfortunately, CLD got worse, the patient remained oxygen-dependent, with intermittent increased RDS. The feeding tolerance was constantly preserved, the baby was bottle-fed with mother milk and fortifiers, with ongoing satisfactory weight gain.

Discussion
Severe osteopenia of prematurity is rare; most of the cases are mild and identified with targeted and specific tests. The diagnosis is rather difficult, usually late, because in the initial phase there is a lack of clinical signs or they are nonspecific and the patients always have more “visible” comorbidities (complications of prematurity affecting vital organs).
Metabolic bone disease could be suspected in a ELBW or VLBW patient with extrauterine growth failure and persistent respiratory distress syndrome, in case of difficulty to wean from ventilatory support, because a low pulmonary volume consecutive to a small and weak thoracic cage with osteopenia generates impaired thoracic compliance and interfere with ventilation.
More severe expression of bone alteration is represented by fractures frequently seen in very premature babies with:
-
NEC (necrotizing enterocolitis)
-
TPN>30 days
-
Cholestasis (malabsorption of vitamin D)
-
Treatment for CLD (chronic lung disease)
-
Prolonged diuretic therapy
-
Aggressive physical therapy.
The incidence of fractures is 2-10% below 29 weeks of gestation, increasing up to 80% if the patient needs prolonged mechanical ventilation and has bronchopulmonary dysplasia (BPD)(2). Usually, fractures are identified at 10-11 weeks of life, symptomatic or by a routine X-ray examination and most often affects ribs, radius, ulna and femur; ribs fractures are associated with higher mortality. The radiographic signs occur late, when 30-40% of the bone mass is lost, or when fractures are already constituted(1).
The gold standard for diagnosis would be DEXA (dual energy X-ray absorptiometry) or QUS (quantitative ultrasound) which measures BMC (bone mass content), but these tests are inaccessible in most of the cases.
The early diagnosis of OP should rely on biochemical markers for bone mineralization which are:
-
Serum calcium level, although it can remain normal for a long period.
-
Serum phosphate level which can be normal/decreased (<3 mg/dl); it has low sensitivity but high specificity.
-
Phosphate serum value has more sensitivity in correlation with increased ALP (alkaline phosphatase).
-
High ALP, especially if increased over time, is a sign of demineralization.
-
ALP over 900 UI/L are predictive for osteopenia of prematurity (sensitivity 88% and specificity 71%), confirmed by DEXA; the sensitivity increases in association with hypophosphatemia (below 1.8 mmol/dl)(3).
-
PTH (parathormone) is decreased/normal.
-
1,25(HO)2 vitamin D is normal or increased.
-
Low urinary calcium/phosphate.
Unfortunately, some specific laboratory tests are not made routinely or are not available at all in some medical centers (calciuria, phosphaturia, vitamin D level, parathormone, calcitonin), so the diagnostic may be delayed. Therefore, the neonatologists should pay attention to epidemiology and risk factors of OP and apply strict diagnostic protocols to identify the process of demineralization and intervene early to correct the metabolic and electrolytic deficits.
The prenatal risk factors for osteopenia of prematurity are considered to be:
-
Gestational age under 32 weeks, as 80% of bone mineralization takes place after 24 weeks of gestation.
-
BW<1500 g is associated with growth factors deficit.
-
Lack of fetal movements against uterine wall resistance is associated with poor growth and mineralization (the mechanostat theory)(4,5).
-
Maternal estrogen level influence fetal bone mineralization.
-
Placental insufficiency.
-
Preeclampsia.
-
Intrauterine growth restriction (IUGR).
-
Chorioamnionitis.
-
Maternal vitamin D, calcium and phosphate deficit.
The postnatal risk factors associated with a high incidence of osteopenia of prematurity are:
-
Nutritional deficits, especially calcium and phosphorus.
-
Total parenteral nutrition (TPN) >30 days.
-
Late enteral feeding or use of unfortified mother milk or inadequate milk formulas.
-
Liquid restriction (usually for PDA).
-
Vitamin D deficit (low intake or malabsorption).
-
Medication – diuretics, steroids, methylxanthines, sodium bicarbonate.
-
Immobilization – sedation, neurologic diseases, sepsis.
-
Other minerals or trace elements deficit – Mg, Cu, Mn, Zn.
-
Chronic lung disease (CLD), necrotizing enterocolitis (NEC), short-gut syndrome, cholestatic jaundice.
The genetic predisposition also seems to play a role. An increased incidence of the disease is seen for:
-
Male sex.
-
Estrogen receptor gene deficit.
-
Collagen alpha 1 gene receptor deficit.
-
Vitamin D receptors gene polymorphism.
Beside mineral intake, other factors interfering with fetal bone mineralization are:
-
Protein and caloric intake.
-
Growth factors IGF1 and IGF2 are decreased in premature and small-for-gestational-age babies.
-
Hormones – insulin, parathormone, calcitonin.
Alcohol use and smoking in pregnancy inhibit fetal bone growth and mineralization(6).
In our case, we can identify as risk factors for a severe form of osteopenia of prematurity the following:
-
Extreme gestational age (24 weeks of gestation).
-
TPN for 39 days without phosphate supplement.
-
Late enteral feeding with mother milk, initially without fortifiers.
-
Diuretic treatment (furosemide) in high doses, for a long period of time.
-
Long-term treatment with steroids and methylxanthines for CLD.
-
Mechanical ventilation for long periods of time, immobilization, sedation.
-
The treatment with Avastin® (two intraocular injections in that period) whose secondary effects in newborns are not fully documented.
-
Antenatal factors (chorioamnionitis, the mother was not prescribed vitamin D3 in pregnancy).
-
Male sex.
In terms of prognosis, the medical literature and our case demonstrate that the clinical course of OP in VLBW and ELBW babies depends very much on the extrauterine growth curve, impaired growth being correlated with poor medium- and long-term outcomes. Even though bone density in premature infants is generally decreased after birth, in adequate conditions of nutrition and care, within six months the Bone Mass Content (BMC) values should become normal for age(1). Former premature infants with OP will still have at 8-12 years old a lower weight, height and BMC compared to former term babies at the same age, and severe cases can lead to impaired somatic development and chronic diseases in adulthood(7). The long-term complications of OP are:
-
myopia due to alterations in skull shape/development
-
dental anomalies (late enamel maturation)
-
lower weight and height
-
lower BMC and lower density
-
predisposition to osteoporosis.
In order to decrease the risk for metabolic disease of prematurity, especially severe, with long-term impairment, the early interventions from pregnancy should be continued postnatally in a sustained and standardized manner.
Fetal and neonatal bone mineralization is influenced by the maternal vitamin D serum level (demonstrated by DEXA – dual energy X-ray absorptiometry). A proper antenatal prophylaxis performed on pregnant women, based on discontinuous administration of calcium and vitamin D, with rational air exposure, especially in the last months of pregnancy, along with avoiding premature births, helps reduce the incidence of osteopenia in premature babies and neonatal hypocalcemia(8).
Vitamin D deficit in pregnancy is associated with a risk of premature birth, SGA newborns, osteopenia and with preeclampsia, insulin resistance and gestational diabetes. The importance of vitamin D as a catalyst for calcium and bone metabolism is well known. It is also involved in the innate immune system and neuromuscular functions. Low levels of vitamin D in umbilical cord blood have been associated with an increased incidence of neonatal sepsis and respiratory tract infections in the first year of life(9).
According to international protocols, a dose of minimum 200 UI/day (10 mcg/day) is recommended for pregnant women, especially if the deficit is documented (below 50 mmol/l or 20 ng/ml)(10).
The transition from intrauterine to extrauterine environment is characterized by hypocalcemia, hypophosphatemia, increasing PTH level, decreasing estrogen level, decreasing mechanical activities, leading to reduction up to 30% of bone density in the first six months of life (the size of medullar channel grow faster than the bone cortex size)(1). Therefore, even in term babies, there exists a physiological osteoporosis, but not accompanied by increased bone fragility, whilst in preterm babies, the reduction of bone density is more rapid and produce increased bone fragility.
The prevention of osteopenia of prematurity in VLBW and ELBW babies is done with enteral/parenteral supplementation with minerals. According to AAP recommendations, the optimal intake of minerals for VLBW is: calcium 150-220 mg/kg/day (50-140 mg/100kcal), phosphorus 70-140 mg/kg/day, and magnesium 7-9 to 15 mg/kg/day (5-15 mg/100kcal).
It is demonstrated that early and high doses of protein administration can lead to an increase of the cellular phosphate uptake and to a decrease of the serum phosphate level in the first weeks of life. The adequate caloric and protein intake is 120 kcal/kg/day and 3-4 g/kg/day proteins.
For the daily practice, it important to know that calcium and phosphate cannot be supplied in the TPN solutions in adequate concentrations for bone mineralization because they precipitate; amino acid solutions with lower pH and Ca-P ratio of 1.3:1 (mg) or 1:1 (mmol) improve the solubility of these minerals in TPN solutions. With all these precautions, the maximum amount of calcium that can be administrated parenterally represents only 60-80% of the intrauterine calcium transfer to the fetus in the last trimester of pregnancy(1).
Beside mineral supplementation, vitamin D3 should be prescribed: 400-1000 UI/day, from the first week of life (even from the first day).
Enteral feeding should start as soon as possible, as parenterally cannot be administrated adequate quantities of minerals. For exclusively enteral fed premature babies, there are recommended orally 2 mmol/kg/phosphate, in order to maintain a phosphate serum level above 6 mg/dL. If phosphate serum level is normal and ALP is increased, calcium supplementation is needed. Studies documented that enteral Ca-P ratio 1.7:1 (mmol) improve intestinal absorption, as minerals tend to adhere to perfusion tubing bolus feeding, allowing a greater delivery of minerals compared with continuous feeding(11).
Prematures with birth weight below 1800-2000 g should receive only fortified mother milk or special milk formulas, for an optimum intake of phosphate. Unfortified mature mother milk has 260 mg/L Ca, 140 mg/L P and 26 mg/L Mg, compared to fortified mother milk, that has 91 mg Ca, 52.5 mg P and 8.1 mg Mg/100 mL, and special milk formulas for preemies with 100 mg Ca, 56 mg P, 8 mg Mg/100 mL. It is important to know that fresh mother milk has more phosphate in comparison with banked donor milk, demonstrated by a study whose outcome was that infants fed with donor pasteurized breast milk had elevated alkaline phosphatase serum level, suggestive for OP(12).
Milk fortifier posology is usually calculated for 100 ml of mother milk; fortification is difficult for smaller volumes. Fortifiers and the administration of milk formulas are indicated until 3000 g or up to 40-52 post-conceptional weeks, even up to 6 months, if there are growth deficits or Rx shows delayed bone mineralization. Sometimes there are necessary protein supplements (PS) – which contain also minerals (calcium – 524 mg/100 g, phosphorus – 516 g/100 g and magnesium – 46 mg/100 g), and this should be taken into consideration.
Attention should be paid when counting nutritive and minerals intake, because too much or too quickly supplementation can generate metabolic or electrolyte imbalances. Excessive supplementation can cause: hypervitaminosis D (multiple sources – milk, fortifier, PS, special preparations), hypervitaminosis A (from fortifiers and PS), hyperglycemia, hypercalcemia, hypercalciuria, nephrocalcinosis.
A recent approach to promote bone mineralization and prevent OP is represented by physical therapy services integrated in neonatal intensive care units, as light daily physical exercises combined with an adequate nutritional intake were demonstrated to be able to improve bone mineralization of the premature babies. Passive mobilization seems to improve weight gain and length and to promote a better bone mineralization, demonstrated by QUS showing improved tibial strength and upper arm circumference in VLBW infants who were included in a kinesiotherapy program. The recommendations include 5-15 minutes of daily exercises: flexion-extension of the extremities, during 3-8 weeks, for stable patients(13). Of course, if the baby is unstable or the biochemical markers for OP are altered, the physical therapy should be recommended with caution and once a fracture is diagnosed, limited movements and maneuvers and immobilization are indicated.
Conclusions
Considering the potential gravity of osteopenia of prematurity in VLBW and ELBW babies and the long-term impact on patients’ health, it is necessary to emphasize that screening programs for the nutritional status of pregnant women and newborns and adequate interventions are proven to be useful and cost effective.
Antenatal care should be focused on vitamin D supplementation for any pregnant woman, especially when there are suspected or documented nutritional deficits or there are other factors that could interfere with vitamin D metabolism. Postnatal care implies to anticipate and prevent OP by early enteral feeding, with the use of special milk formulas or fortified fresh mother milk and protein supplements, phosphate supplementation for parenteral nutrition, adequate and early vitamin D administration for newborns (per os or intravenous) and weekly monitoring of serum Ca, P and ALP for all VLBW/ELBW premature babies, from 2 weeks of age, continued even after discharge, if the signs of demineralization persist. The interdisciplinary approach, from obstetrician to neonatologist, in collaboration with other specialists (radiology, pediatrics, specialists in the field of physical therapy), along with integrated and adequate interventions are the key to successfully overcome this complication of prematurity.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Fanaroff AA, Martins EJ. Neonatal Perinatal Medicine. Diseases of the fetus and infant. Disorders of Calcium, Phosphorus and Magnesium metabolism. In: Neonate, Osteopenia of Prematurity, 10th Ed. 2015:1460-88.
-
Binder C, Wild J, Thanhaeuser M, Repa A, Kreissl A, et al. Osteopenic fractures in Preterm Infants < 1500 grams. A retrospective data analysis over 10 years at the Medical University of Vienna. J Pediatr Neonatal Care. 2015;2(2):00063.
-
Backström MC, Kouri T, Kuusela AL, et al. Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr. 2000;89(7):867-873.
-
Jee WS, Frost HM. Skeletal adaptations during growth. Triangle. 1992;31(2/3):77-88.
-
Rauch E, Schoenau E. Skeletal development in premature infants: A review of bone physiology eyond nutritional aspects. Arch Dis Child Fetal Neonatal Ed. 2002;86:F82-F85.
-
Gomella’s Neonatology. Management, procedures, on call problems, diseases, and drugs, 8th Ed., 2020;1022-6.
-
Fewtrell MS, Cole TJ, Bishop NJ, et al. Neonatal factors predicting childhood height in preterm infants; evidence for a persisting effect of early metabolic bone disease. J Pediatr. 2000;137:668-73.
-
Voloc A, Ţurea V, Rotari A. Particularităţile clinice, de diagnostic şi de tratament al stărilor hipocalcemice la copil. Bul Perinatol. 2008;N4(40):39-44.
-
Cooper C, Eriksson J, Forsen T, et al. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporosis Int. 2001;12(8):623–9.
-
American Academy of Paediatrics. Statement of Endorsement: Dietary reference intakes for Calcium and vitamin D. Paediatrics. 2012;130(5):e1424.
-
Fanaroff AA, Martin EJ. Neonatal Perinatal Medicine. Diseases of the fetus and infant, 6th Ed, 1997:1473-6.
-
Kazmi SH, Berman S, Caprio M, Wachtel EV. The impact of donor breast milk on metabolic bone disease, postnatal growth and neurodevelopmental outcomes at 18 months corrected age. J Parent Enteral Nutr. 2022;46(3):600-7.
-
Schultzke S, Kaempfen S, Trachsel D, Patole S. Physical activity programs for promoting bone mineralization and growth in preterm infants. Cochrane Database Syst Rev. 2014;38:1685-90.