The diagnosis and treatment of acute myeloid leukemia (AML) require an integrated and complete approach that takes into account clinical and laboratory data, the morphological evaluation of the marrow aspirate and peripheral blood, immunophenotyping, cytogenetic evaluation, and molecular analysis. The identification of these data has the role of stratifying patients into risk categories, intended to guide the intensity and type of treatment indicated for each individual case, doubled by the possibility of identifying
mutations with a direct therapeutic effect (FLT3, NPM1). Taking into account the classification of acute myeloid leukemias, similarities of the proposed new entities and a trend towards ICC-WHO harmonization can be observed, the aim being to create a unified model between the existing sets of recommendations.
A comparative approach to classifications in the diagnosis of acute myeloblastic leukemia with reference to cytogenetic, myelodysplastic elements and mutations of the TP53 gene – WHO, ICC, ELN 2022
O abordare comparativă a clasificărilor în diagnosticul leucemiilor acute mieloblastice, cu referire la elemente citogenetice, mielodisplazice şi mutaţii ale genei TP53 – OMS, ICC, ELN 202
First published: 27 martie 2024
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.66.1.2024.9389
Abstract
Rezumat
Diagnosticul şi tratamentul leucemiei mieloide acute presupun o abordare integrată şi completă ce ţine cont de datele clinice şi de laborator, de evaluarea morfologică a aspiratului medular şi a sângelui periferic, incluzând imunofenotiparea, de evaluarea
citogenetică şi analiza moleculară. Identificarea acestor date are rolul de a stratifica pacienţii în categorii de risc, menite să ghideze intensitatea şi tipul tratamentului indicat fiecărui caz în parte, dublate de posibilitatea evidenţierii unor mutaţii cu viză terapeutică directă (FLT3, NPM1). În ceea ce priveşte clasificarea leucemiilor acute mieloide, se pot observa similarităţi între noile entităţi propuse şi tendinţa de armonizare ICC-OMS, scopul fiind crearea unui model unificat între seturile de recomandări existente.
The current classification systems of acute myeloid leukemias, which divide these entities into multiple subcategories, are based on the integration of specific genetic mutations, selectively incorporating the percentage of blasts. Their use in current practice gives clinicians decision-making support in the diagnosis and therapeutic approach towards patients.
In the classification of acute myeloblastic leukemia (AML), chromosomal rearrangements are well established. In 2022, new WHO (World Health Organization)(2) and ICC (International Consensus Classification)(1,6) classification schemes were described, together with the ELN recommendations(4) related to its diagnosis and management.
Taking into account the biological impact of certain chromosomal aberrations and the latest mutational changes described, the WHO classification(5) describes a variety of changes that can characterize acute leukemias, defining the subtypes of AML both in terms of recurrent genetic abnormalities and by the stage of differentiation, when no characteristic genetic alterations are evident. In 2022, the WHO classification eliminated the percentage of 20% of blasts necessary for the diagnosis of AML for all cases with defined genetic abnormalities (defining genetic abnormalities), with the exception of AML with BCR::ABL1 fusion, AML with CEBPA mutation, and with other rare genetic changes defined which still require the percentage of 20% blasts (Table 1). The changes are based on studies which demonstrated that patients with these cytogenetic abnormalities and with a percentage of blasts below 20%, defined in 2017 as myelodysplastic syndrome, had a clinical evolution similar to that of patients with a high percentage of blasts, and the progression to acute myeloid leukemia was rapid. ICC 2022 imposes a percentage of blasts of 10% along with the recurrent genetic anomalies mentioned in Table 1, with the exception of the BCR::ABL1 fusion gene, in which the percentage of 20% persists in both systems to be able to differentiate it from the accelerated phase of chronic myeloid leukemia.
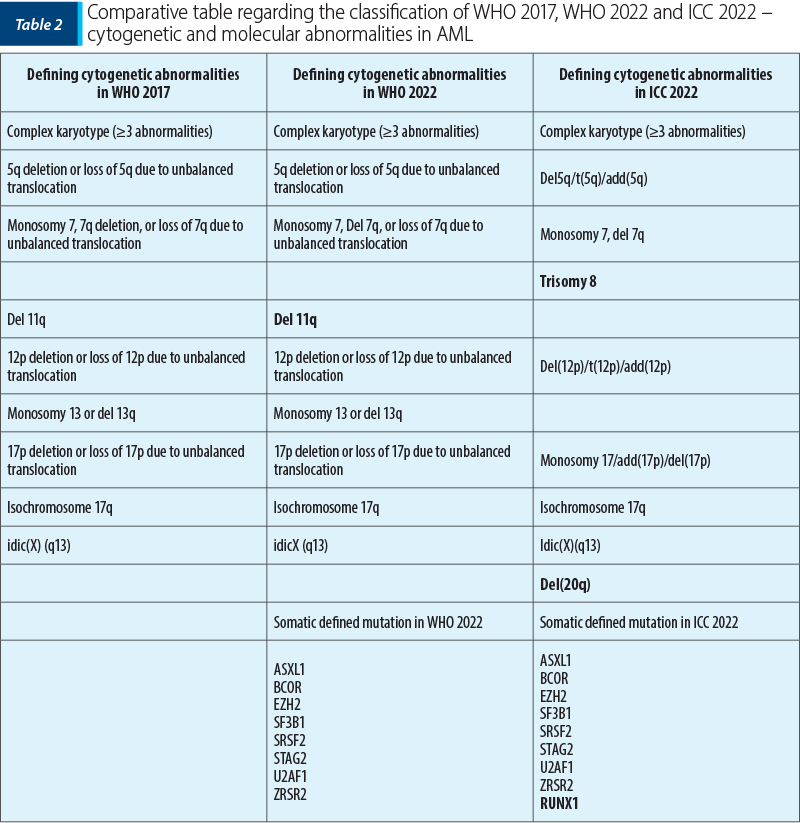
The genetic abnormalities defined for AML in WHO 2022 and the recurrent genetic abnormalities in ICC 2022 are similar to those listed by WHO in 2017, with small differences, mentioned in Table 1.

In the case of CEBPA gene mutations (CCAAT enhancer binding protein A gene), WHO 2022 involves biallelic mutations (biCEBPA) and single mutations located in the basic leucine zipper region of the smbZIP-CEBPA gene, while ICC 2022 includes only in-frame bZIP; recent studies consider that a favorable prognosis is associated with patients with CEBPA in-frame bZIP mutations, regardless of their appearance as biallelic or monoallelic mutations(7,8-10).
The entity AML with RUNX1 mutation from WHO 2017 was reclassified into subtypes in both classifications (WHO 2022 and ICC 2022), as follows:
- 74.4% as AML with myelodysplasia-related changes (AML-MR)
- 22% as AML with differentiation-related changes (AML-DD)
- 2.4% as AML with mutated CEBPA (AML-CEBPA)
- 1.2% as AML with MECOM rearrangements (AML-MECOM).
The classification of high-grade myelodysplastic syndromes (high-grade MDS) represents another point of divergence between the two classification systems of acute myeloid leukemias.
A series of updates was brought to the AML entity with changes related to myelodysplasia (AML-MR; myelodysplasia-related changes), which in 2022 was renamed as AML-MR myelodysplasia related, and it was divided into two subcategories by the ICC: acute myeloid leukemia acute with genetic mutations related to myelodysplasia (ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1 or ZRSR2) and acute myeloid leukemia with cytogenetic abnormalities related to myelodysplasia. Morphologically, the required percentage of described blasts is ≥20%. A history of myelodysplastic syndrome or myeloproliferative neoplasm represents a descriptive condition in the diagnosis, but it does not represent a classification criterion. Both the new WHO classification and the ICC guide emphasize the genetic lesions associated with myelodysplasia in AML, as part of the ontogenetic process. However, the framing of these genetic anomalies cannot be superimposed in these two systems, leading to discrepancies in the clinical management of patients.
Cytogenetic and molecular abnormalities in acute myeloid leukemia related to myelodysplasia (AML-MR; myelodysplasia related; WHO 2017/2022 and ICC 2022)
An important category, mentioned by ICC, is given by acute myeloid leukemia with mutant TP53. To support this diagnosis, it is necessary that the percentage of blasts be at least 20% and that there is any TP53 somatic mutation at the level of a varied allelic fraction of more than 10%; if the percentage of blasts was between 10% and 19%, then it would be classified as SMD/LAM with mutated TP53. The prognosis of these patients is unfavorable, presenting numerous anomalies, sometimes being associated with a complex karyotype(11-15). Due to the frequent correlation of pure erythroid leukemia with TP53, in ICC these cases will be classified as acute myeloid leukemia with mutant TP53.
In the case of patients with a percentage between 10% and 19% blasts in the periphery and/or bone marrow, a new category was introduced by ICC: myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), replacing MDS with excess blasts, grade 2 (WHO 2017), thus creating the opportunity for patients to participate in clinical trials (MDS or AML). WHO 2022 continues to classify these patients as having myelodysplastic syndrome with increased number of blasts grade 2 (MDS-IB2; increased blasts 2), name changed to MDS with excess blasts, a measure taken to avoid overtreatment, although WHO mentions that MDS-IB2 can be considered AML for therapeutic purposes, if it has a clinical indication.
It has been shown that the incidence of AML cases in patients with neoplastic history is increasing, currently representing 10-15% of all newly diagnosed AML(16). It is considered that these neoplasms are the direct consequence of mutational events induced by cytotoxic therapy and/or the selection of clones resistant to chemotherapy(17-19). In 2022, WHO divided myeloid neoplasms secondary to exposure to cytotoxic therapy or germline predisposition in the major category of secondary myeloid neoplasia with three subcategories: post-cytotoxic therapy myeloid neoplasia (previously called therapy-related AML), MN-pCT – myeloid neoplasms associated with germline predisposition, and myeloid proliferation associated with Down syndrome.
According to the ELN 2022 classification, the FLT3-ITD mutation is part of the intermediate risk group, regardless of the allelic ratio or the concomitant presence of NPM1. AML with gene mutations related to myelodysplasia is now included in the unfavorable risk group.
ELN classification of the genetic risk group at diagnosis in 2022
Favorable:
- t(8;21)(q22;q22.1)/RUNX1::RUNX1T1.
- inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/CBFB::MYH11.
- NPM1 mutant without FLT3-ITD.
- bZIP in-frame mutated CEBPA.
Intermediate:
- NPM1 mutant with FLT3-ITD.
- Wild-type NPM1 with FLT3-ITD (without genetic lesions with adverse risk).
- t(9;11)(p21.3;q23.3)/MLLT3::KMT2A.
- Cytogenetic and/or molecular anomalies not classified as favorable or adverse.
Unfavorable:
- t(6;9)(p23.3;q34.1)/DEK::NUP214.
- t(v;11q23.3)/KMT2A – rearranged#.
- t(9;22)(q34.1;q11.2)/BCR::ABL1.
- t(8;16)(p11.2;p13.3)/KAT6A::CREBBP.
- inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/GATA2, MECOM(EVI1).
- t(3q26.2;v)/MECOM(EVI1) – rearranged.
- −5 or del(5q); −7; −17/abn(17p).
- Complex karyotype, monosomal karyotype.
- ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, and/or ZRSR2*mutant.
- TP53 mutant (VAF at least 10%).
Monosomal karyotype = the presence of a single monosomy (except loss of X or Y chromosomes) in association with at least one more monosomy or another chromosomal abnormality (except core-binding factor).
Complex karyotype = the presence of three or more chromosomal anomalies, unrelated in the absence of other recurrent genetic anomalies, define a class, exclude hyperploid karyotypes with three or more trisomies, without structural anomalies.
* if they are associated with mutations in the category of those with a favorable prognosis, they are no longer factors of an unfavorable prognosis;
# it is frequently associated with the complex or monosomal karyotype.
The correct and complete diagnosis of AML is important for obtaining complete remission, and with the help of targeted therapy (anti-FLT3, IDH1, IDH2, BCL2) or menin inhibitors (KMT2 rearrangement, NPM1 mutation)(20-23). At the same time, based on the cytogenetic and molecular genetic characteristics at diagnosis and the response to treatment, the indication for hematopoietic cell transplantation, the only method of curative therapy will be decided. Cytogenetic evaluation of patients with AML at the time of diagnosis is important in order to obtain complete remission and maintain it, thus ensuring an increased quality of life for the patient.
The development and implementation of a unified classification system require a critical and permanent review of the current literature, as well as the integration of new investigations available in the field of acute myeloproliferations. The initiative is shaped and supported by multiple international consortia whose area of research specifically targets MDS and AMLs, seeking a patient-centered approach to care and better clinical integration of cohort data from the literature. An ideal model for a classification system will require continuous involvement and harmonization of the existing data.
Corresponding author: Meilin Omer E-mail: meilin_26@yahoo.com
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200-1228.
-
Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36(7):1703-1719.
-
Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377.
-
Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447.
-
Heuser M, Freeman SD, Ossenkoppele GJ, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138(26):2753-2767.
-
Arber DA, Hasserjian RP, Orazi A, et al. Classification of myeloid neoplasms/acute leukemia: Global perspectives and the international consensus classification approach. Am J Hematol. 2022;97(5):514-518.
-
Tarlock K, Lamble AJ, Wang YC, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the Children’s Oncology Group [published correction appears in Blood. 2022;139(10):1601]. Blood. 2021;138(13):1137-1147.
-
Sierra J, Nomdedeu JF. CEBPA bZip mutations: just a single shot. Blood. 2021;138(13):1091-1092.
-
Taube F, Georgi JA, Kramer M, et al. Study Alliance Leukemia (SAL). CEBPA mutations in 4708 patients with acute myeloid leukemia: differential impact of bZIP and TAD mutations on outcome. Blood. 2022;139(1):87-103.
-
Wakita S, Sakaguchi M, Oh I, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022;6(1):238-247.
-
Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221.
-
Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114-2121.
-
Middeke JM, Herold S, Rücker-Braun E, et al. Study Alliance Leukaemia (SAL). TP53 mutation in patients with high-risk acute myeloid leukaemia treated with allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2016;172(6):914-922.
-
Montalban-Bravo G, Benton CB, Wang SA, et al. More than 1 TP53 abnormality is a dominant characteristic of pure erythroid leukemia. Blood. 2017;129(18):2584-2587.
-
Short NJ, Montalban-Bravo G, Hwang H, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv. 2020;4(22):5681-5689.
-
Morton LM, Dores GM, Schonfeld SJ, et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019;5(3):318-325.
-
Voso MT, Falconi G, Fabiani E. What’s new in the pathogenesis and treatment of therapy-related myeloid neoplasms. Blood. 2021;138(9):749-757.
-
McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17(9):513-527.
-
Schwartz JR, Ma J, Kamens J, et al. The acquisition of molecular drivers in pediatric therapy-related myeloid neoplasms. Nat Commun. 2021;12(1):985.
-
Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740.
-
DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398.
-
Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
-
Stein EM, Aldoss J, DiPersio JF, et al. Safety and efficacy of menin inhibition in patients with MLL-rearranged and NPM1 mutant acute leukemia: a phase 1, first-in-human study of SNDX-5613. Blood. 2021;138(Suppl 1):699.
Articole din ediţiile anterioare
Clasificarea moleculară a cancerelor colorectale şi importanţa ei clinică - scurt review
Cancerul colorectal (CRC) reprezintă unul dintre cele mai frecvente tipuri de cancer, fiind caracterizat de alterarea căilor critice, cum ar fi: WN...
Mastocitoza – o patologie unică, având multiple faţete
Mastocitoza este o boală rară care a fost definită ca o acumulare anormală de mastocite în unul sau mai multe sisteme de organe. Anterior...
New changes in the classification of acute myeloid leukemia proposed by WHO 2022
Noua revizuire a clasificării OMS (Organizaţia Mondială a Sănătăţii) din 2022 a leucemiilor acute mieloide (LAM) are ca scop evidenţierea noilor da...