Triple negative breast cancer (TNBC) has a poor prognosis even in early stages, denoting a 5-year relapse rate of 30% in stage I to III of disease, with less than one third of patients being alive at 5 years after relapse event. There is no validated maintenance adjuvant treatment in hormone negative, HER2-negative breast cancer phenotype. Metronomic chemotherapy (MT), chronic administration of cytotoxic agents in low doses, confers the advantage of reduced toxicities and of an antiangiogenic and immunomodulatory complementary effect. Running a general review on the benefit of adjuvant MT in operable TNBC population, capecitabine, cyclophosphamide, methotrexate and vinorelbine, either in monotherapy or in combination, were found most studied. Generally, this schedule was well tolerated even in one-year administration regimen. In two Egyptian phases II studies exploring the safety of capecitabine, the administration for six months, respectively one year, lead to an estimated median disease free survival (DFS) of 42.4 months (95% CI [39.02-45.79]) respectively 41.7 months (95% CI [36.5-46.9]). However, phase III studies reported grade III-IV adverse events in near a quarter of patients for 8 cycles of capecitabine standard dose after adjuvant therapy and no survival benefit of one-year cyclophosphamide-methotrexate metronomic compared to standard approach. Several ongoing phase III studies evaluate cyclophosphamide efficacy for six months to one year, following standard treatment. Metronomic adjuvant maintenance treatment seems feasible in terms of toxicity in operable TNBC. Survival benefit remains to be proven.
Chimioterapia metronomică în cancerul mamar triplu negativ operabil
Metronomic chemotherapy in operable triple negative breast cancer
First published: 07 martie 2017
Editorial Group: MEDICHUB MEDIA
Abstract
Rezumat
Cancerul mamar triplu negativ reprezintă un fenotip tumoral de prognostic defavorabil chiar şi în stadiile iniţiale, prezentând o rată de recidivă de 30% în stadiile I-III şi o supravieţuire la 5 ani mai mică de o treime în rândul pacientelor care recidivează. În cancerul mamar fără expresia receptorilor hormonali sau HER2, nu este validat un tratament adjuvant de susţinere. Chimioterapia metronomică (CM) presupune administrarea cronică de doze mici de agenţi citostatici, conferind avantajele unui profil de toxicitate scăzut şi ale unei acţiuni complementare antiangiogenice şi imunomodulatoare. Procedând la un review al literaturii publicate asupra beneficiului CM ca tratament adjuvant în cancerul mamar triplu negativ operabil, capecitabina, ciclofosfamida, metrotexatul şi vinorelbina au fost găsite ca fiind cele mai studiate, fie în monoterapie, fie în combinaţie. În general, toleranţa a fost bună chiar şi pentru regimuri cu durata de un an. În două studii egiptene de fază II, în care s-a explorat toleranţa la administratea capecitabinei pe o durată de 6 luni, respectiv 1 an, supravieţuirea mediană (DFS) a fost 42,4 luni (95% CI [39,02-45,79]), respectiv 41,7 luni (95% CI [36,5-46,9]). În studii de fază III, evaluând 8 cicluri de capecitabină adjuvantă administrată în doză standard, au fost raportate toxicităţi de grad III-IV la aproape un sfert din cazuri şi nu s-a înregistrat nici un benficiu în supravieţuire, faţă de conduita standard. Actualmente, mai multe studii în curs de fază III evaluează eficienţa ciclofosfamidei în regimuri de 6 luni până la 1 an. Tratamentul citostatic metronomic pare fezabil ca tratament de susţinere de lungă durată în privinţa toxicităţii. Beneficiul clinic ca tratament adjuvant în cancerul mamar TN operabil rămâne de dovedit.
Introduction
Breast cancer is the leading cause of cancer-related death in women(1). It is a heterogeneous disease comprising different subtypes according to estrogen, progesterone and human epidermal growth factor 2 receptors expression which varies in terms of morphology, biology and prognostic(2).International adopted classification, according to immunohistochemical staining of estrogen, progesterone and human epidermal growth factor 2 receptors and KI67 proliferation marker distinguishes four principal entities of breast cancer, of which two expressing hormone receptors (luminal A, luminal B), one only HER2 (HER2 positive) and one highly proliferative, negative for all specific receptors (triple negative)(3).
Triple negative breast cancer (TNBC) accounts for 10-20% of all invasive breast cancers(4) and is defined by American Society of Clinical Oncology/College of American Pathology (ASCO/CAP) panel as less than 1 percent staining of tumor cells by IHC for hormone receptors either 0-1+ or 2+ score and fluorescence in situ hybridization (FISH)-negative for HER 2(5). It represents a surrogate for basal-like subtype defined as microarray expression of genes usually found in myoepithelial cells of the breast, including those encoding basal cytokeratin (CK) 5/6, 14 and 17 which are normally found in the basal layer of stratified epithelium, representing about 75% of triple negative(1,6).
General characteristics of TNBC are the prevalence in young age, large tumor size, high histological and proliferation rate, pushing border of invasion and high rate of advanced stages at diagnostic(7).
Prognosis is poor even in early stages: 5-year relapse rate in stage I-III patients, that underwent adjuvant chemotherapy, is about 30%, the median time to metastatic recurrence is less than 3 years and the 5-year survival rate after the metastatic event is less than 30%(8).The risk to develop visceral metastases is three times higher than in other types of breast cancer, frequently in lung and brain(9). In very early stages, T1N0-1, 5-year disease free survival (DFS) and cancer-specific survival are approximately 85%(10).
Highly proliferative tumors, such as TNBC, have an enhanced angiogenesis that supports rapid growth and early metastasis, being correlated with levels of vascular endothelial growth factor(11). Inside TNBC by gene expressing analysis, it has been identified a mesenchymal stem-like (MSL) subtype enriched in genes involved in angiogenesis and one immunomodulatory (IM) highly enriched in gene involved in immune cell processes, markers and signaling, the last one being found in 20% of cases(12).
In localized disease, in the absence of validated maintenance adjuvant treatment, the cytotoxic chemotherapy is still the mainstay for TNBC, despite the promise of new targeted and biologic agents(13).
Metronomic chemotherapy (MT) refers to the chronic administration of low doses of cytotoxic drugs at close and regular intervals with no prolonged drug-free interruptions(14). Low-doses schedule was found to affect not only tumor cells, but also their microenvironment and repairing process of endothelial cells, leading to an anti-angiogenic effect(15).
In vitro studies showed that low dose of cyclophosphamide results in cytoskeleton alteration, enhancement of antioagiogenic factors expression (such as TSP-1) or induction of pro-apoptotic signal (NF-kB)(16).
In addition, MT exerts an immunological action through the restoration of the anticancer effect of immune system(17).
MT efficacy has shown promising activity in metastatic setting in several solid tumors including breast cancer(18). By general low profile toxicity, this schedule showed to be feasible for long term administration including in adjuvant setting(19).
Thus, the setting of metronomic chemotherapy in operable TNBC seems to be adjuvant either after neoadjuvant chemotherapy (CHT) especially in non pCR, either in continuation of standard adjuvant treatment.
A general review about the role that MT might have as adjuvant treatment in operable triple negative breast cancer was herein conducted.
The role of metronomic chemotherapy
Most studied anticancer agents as metronomic schedule, in breast cancer, belong to alkylating agents class that interact with DNA molecules (cyclophosphamide), antimetabolites, interfering with DNA and RNA synthesis (methotrexate, capecitabine) and vinca alcaloids that blocks microtubules formations, cancer cells accumulation in mitosis and deaths by apoptosis(20).In vitro exposure to 4-hydroxi-cyclophospmide showed that high doses have a direct cytotoxic activity, whereas the lowest doses inhibit the endothelial proliferation(21). In a prospective observational study (n=63) serum VEGF level drop down below to median baseline value was significantly correlated with clinical benefit of low-dose oral methotrexate (MTX) and cyclophosphamide (CTX) in patients with metastatic breast cancer(22).
Low doses of CTX have also been shown to modulate tumor microenvironment cells, including pericytes and tumor infiltrating lymphoid cells(23). Furthermore, several immunological effects have been reported. In mice models, metronomic cytotoxic administration induces a decrease in level of several immunosupresive cytokines, such as TGF-b, IL10, and IL2(24). Dendritic cells maturation is also stimulated, resulting in prolonged memory T cells survival(25). Low doses of CTX showed to stimulate Galactin-1 expression, a down regulator of cells proliferation and dendritic cells differentiator(26).
Generally used metronomic regimens
In breast cancer, metronomic regimens were firstly used and related ongoing studies are mainly conducted in metastatic setting.Cyclophosphamide is the first cytotoxic drug studied in MT administration in pretreated metastatic breast cancer (MBC) patients, by Colleoni et al., in 2002. The dose was fifty milligrams daily associating methotrexate, 2.5 mg twice daily, 2 days per week. Clinical benefit initially reported was of 24 weeks with no grade 3, 4 adverse reactions(27). A subsequent report showed a median time to progression in patients with prolonged clinical benefit of 21 months, ranging from 12 to 37 months(28).
Other anticancer agents tested in low doses continuous schedules, either in monotherapy or in combination, are capecitabine and vinorelbine.
Capecitabine in low doses, continuously 650 mg/m2 - 800 mg/m2 bid, seems to be similar in terms of efficacy to standard regimen either in pretreated or in first line MBC(29).
In sixty heavily treated patients, 1500 mg capecitabine single daily dose led to a clinical benefit (CB) of 62% and an overall survival (OS) of 17 months. Grade 3 toxicities, hand and foot syndrome and neutropenia were described in less than 10% of cases(30).
The pharmacokinetic profile permitted vinorelbine oral formulation to be administrated in metronomic schedules. In monotherapy in non-treated elderly patients (n=34) a weekly dose of 70 mg/m2, fractionated on days 1, 3, and 5, for 3 weeks of 4, secured a 38% overall response rate (ORR), a median progression free survival (PFS) and median OS of 7.7 and 15.9 months respectively(31).
Due to the observed in vitro synergetic effect of aforementioned cytotoxic agents, combinations in metronomic schedule have been proposed. In addition to clinical benefit reported by Calleoni and colleagues, a phase II study testing cyclophosphamide - metotrexat association found a good tolerability even in pretreated patients(32).
Dose limiting toxicity (DLT) for capecitabine - vinorelbine association was reached at 70 mg for oral metronomic vinorelbine and at 1250 mg/m2 for capecitabine(33). CB reported for a regimen of fixed dose of capecitabine (500 mg three times a day) and vinorelbine (40 mg three times a week) was of 58.1% in 31 pretreated MBC(34).
Even though the chemotherapy - hormonal combination isn’t feasible because of contradictory antitumor mechanism, metronomic schedules acting mainly through antiangiogenic mechanism and less through cytotoxic activity might allow the concomitant administration of the two classes of anticancer agents(35). In a phase II study, Schwartzberg et al. explored the activity of fulvestrant and low-dose metronomic capecitabine in 41 MBC patients with positive hormone receptor and negative HER2. The median duration of therapy was 11 months, with a median PFS of 14.98 months, and a median time to progression (TTP) of 26.94 months, and the tolerance was good(36). Safety and antitumor activity of a cyclophosphamide - megestrol acetate combination was evaluated in twenty-nine pretreated post-menopausal MBC patients. The treatment consisted in cyclophosphamide 50 mg daily, days 1-21 of 28 and fractionated megestrol acetate 80 mg twice a day. The overall ORR was 31% and the mean OS was 13.4 months(37).
Antiangiogenic effect of MC might be synergetic with targeted approved agents. In HER2 overexpressed or amplified small sample population (n=22), the combination of trastuzumab and metronomic CM in trastuzumab pretreated MBC patients, the CB was 46% and the median time to progression was 6 months(38).
Regarding bevacizumab, added to metronomic cyclophosphamide containing regimen, in pretreated population, allowed a 63% clinical benefit (CB), with a mild toxicity(39). In non-pretreated triple negative breast cancer, a phase II study, bevacizumab combined with erlotinib along with cyclophosphamide and capecitabine combination reported (n=24) an ORR of 62% and a CB of 75%. Grade 3 toxicity, including diarrhea, thrombosis and hypertension, was reported in 16% of cases(40).
In 2014, at the ASCO meeting, the results of a randomized phase III trial of bevacizumab therapy with either weekly paclitaxel or daily oral metronomic capecitabine and cyclophosphamide as first-line treatment in 147 HER2-negative MBC patients showed no difference in term of ORR (58% vs. 50%, p=0.45) or incidence of grade 3-5 adverse events (25% vs. 24%). Nevertheless, a statistically difference has been reported regarding the quality of life in favor of metronomic regimen(41).
The metronomic chemotherapy in non-metastatic TNBC
In operable triple negative breast cancer, due to very low level of expression of estrogen, progesterone and HER2 receptors, cytotoxic chemotherapy remains the mainstay and there is no validated maintenance adjuvant treatment(42).Several metronomic regimens have been evaluated for safety and benefit in adjuvant setting, in early stages of TNBC. Two years of uracil tegafur (UFT), an oral fluropirimidine approved in metastatic colorectal cancer, assessed as adjuvant treatment, was found as effective as 6 cycles of classic CMF in high risk breast cancer, node negative population, primary treated by surgery: OS 96.2% vs 96%, HR for RFS 0.98 (p=0.92). Stratification along hormonal receptor status was preplanned and most of the patients had invasive ductal histology (>90%), T2 stage, in both arms. In ER-PgR- population, hazard ratio was in favor of CMF regimen (HR: 1, 1 95% CI [0,6-2,2](43).
The two-year analysis of a phase III study, early closed due to inadequate accrual, showed that in older cancer patients with operable ER-PgR - breast cancer, 16-week adjuvant metronomic cyclophosphamide and methotrexate was associated with a better quality of life and cognitive function as compared to pegylated liposomal doxorubicin (PLD). At a median follow-up of 42 months, 19% of the 77 enrolled patients experienced a breast cancer related recurrence. The 3-year Kaplan-Meier estimate of breast cancer free interval was similar for the PLD and non-PLD group of 78%(44).
Always referring to elderly patients, when single-agent capecitabine adjuvant treatment was compared to standard chemotherapy (classical CMF or AC) in the CALGB49907 trial, which included women aged 65 years or older with early-stage breast cancer, it was found inferior in terms of survival. Among patients with hormone-receptor-negative tumors who received capecitabine, the risk of relapse was more than quadrupled (HR 4.39, 95% CI [2.9 - 6.7], p<0.001) and the risk of death was more than tripled (HR 3.76, 95% CI [2.23 - 6.34] p<0.001), as compared with patients in all other study groups combined(45).
Based on the synergism observed in xenograft models between cyclophosphamide, docetaxel and capecitabine, the FINXX trial questioned the benefit of adding fluoropirimidine to three cycles of docetaxel (TX) followed by three cycles of cyclophosphamide, epirubicin and capecitabine (CEX) in axillary node-positive or high-risk node-negative breast cancer patients(46). Capecitabine schedule was 900 mg/m2 given twice daily on day 1 to day 15 every 3 weeks. In a post hoc exploratory analysis, TX/CEX sequence was more effective than T/CEF (HR= 0.48; 95% CI [0.26-0.88], p=0.018) in triple negative subgroup. Regarding the regimen’s safety, four of six deaths reported in experimental arm were treatment-related. Treatment discontinuation was more often recorded in patients assigned to receive capecitabine: 24% (178 patients) versus 3% (23 patients), p=0.001(47).
At SABC 2010, there have been presented the first safety data from a large randomize trial (n=816) assessing the survival benefit of capecitabine maintenance therapy after standard treatment in early stages of TNBC. The preliminary report in 400 patients who have received 8 cycles of capecitabine 1000 mg/m2 twice daily, for fourteen days, every three weeks, found 25.7% of experimental related grade 3-4 adverse events of which 17.4% grade 3 hand-foot syndrome. The planned dose was administrated in 77.3% of cases(48).
Two Egyptian phase II studies explored the safety of adjuvant extended administration of capecitabine in adjuvant setting in specific population. In one study conducted between June 2010 and December 2013, in 41 early TNBC, capecitabine was administrated continuously for six months, 500 mg PO twice daily, following standard adjuvant treatment (six cycles of adjuvant FEC100 ± postoperative radiotherapy). The tolerance was good, the adverse events registered being only of grade I. Survival estimations were also appealing, mean DFS being of 42.4 months (95% CI [39.02 - 45.79]) and mean OS of 44.34 months (95% [CI 41.9 - 46.9])(49). The aim of the other study was the efficacy of 1 year of oral metronomic capecitabine (650 mg/m2, twice every day), after completion of standard adjuvant treatment. In nineteen TNBC patients, the median DFS was 41.7 months ± 2.7 (95% CI [36.5 - 46.9]), the actuarial rate of DFS was 88.8% and 82.05% at 2 and 3 years respectively. Related to safety, there was reported only one case of grade 3 hand-foot syndrome and one case of diarrhea. All patients completed the proposed treatment without dose reduction(50).
Recently, the results of a phase III trial have been published, that evaluated the efficacy of 12 months of maintenance of cyclophosphamide (50 mg/day) and methotrexate (2.5 mg/twice a day orally, days 1 and 2 of every week) in early breast cancer patients displaying less than 10% staining for estrogen and progesterone receptor in tumor cells. After a median follow-up of 6.9 months, DFS wasn’t found significant either in the experimental group - HR=0.84 (95% CI [0.66 -1.06], p =0.14) - or in TN patients, HR=0.72 (95% CI [0.49 - 1.05]. Grade 3 or 4 treatment-related adverse events were reported in 14% (64 patients) of cases, of which elevated serum transaminases being the most frequently reported (7%), followed by leukopenia (2%)(51).
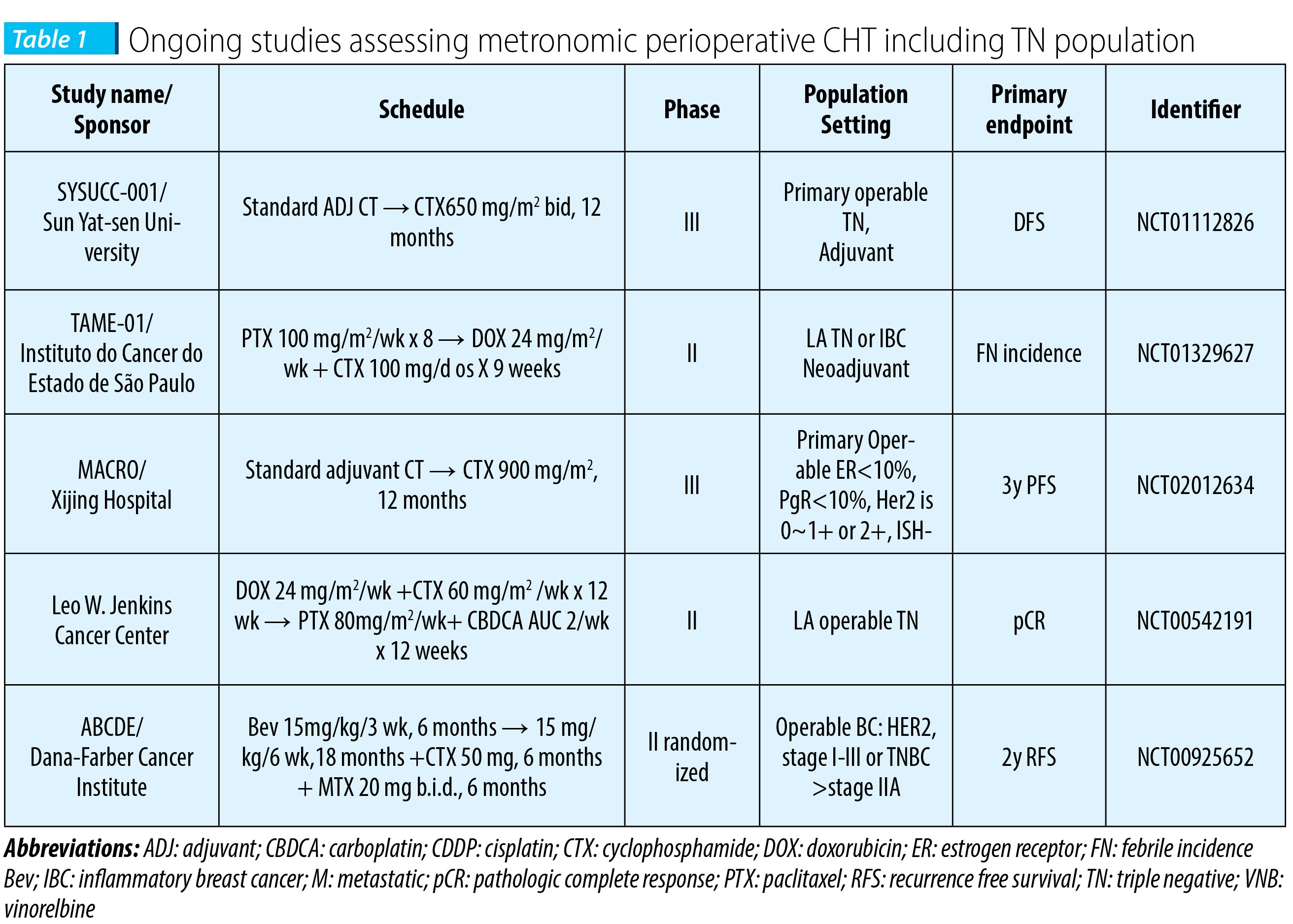
Several ongoing phase II-III studies are testing perioperative MT, specific in operable TN population, beyond standard adjuvant treatment, mostly comprising CTX. The results of a pilot study, assessing one year of adjuvant bevacizumab with or without cyclophosphamide and methotrexate in women with operable breast cancer, are pending (Harold J. Burstein, MD, PhD NCT00121134).
Conclusions
Metronomic maintenance chemotherapy is feasible in terms of toxicities to be administrated in adjuvant setting in operable triple negative breast cancer, following standard treatment.There is an important need of adjuvant maintenance treatment in non-metastatic hormone-negative, HER2-negative breast cancer.
Survival benefit of this approach is still under investigation and remains to be proven.
In the era of immunotherapy, metronomic chemotherapy, through its immunomodulatory mechanism, might be an affordable surrogate for this type of treatment. n
Conflict of interest: The author declares no conflict of interest.
Bibliografie
2. Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5:5–23.
3. Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546.
4. Dent R, Trudeau M, Pritchard KI et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007 Aug 1;13[15]:4429-34.
5. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28[16]:2784.
6. Cheang MC, Martin M, Nielsen TO et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. 2015 May;20[5]:474-82.
7. Azim HA Jr, Michiels S, Bedard PL et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012 Mar 1;18[5]:1341-51.
8. Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast cancer research and treatment. 2009;113[2]:357-70.
9. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul 12;384[9938]:164-72.
10. Lai HW, Kuo SJ, Chen LS et al. Prognostic significance of triple negative breast cancer at tumor size 1 cm and smaller. Eur J Surg Oncol. 2011 Jan;37[1]:18-24.
11. Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiç J, et al. Significantly higher levels of vascular endothelial growth factor [VEGF] and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol 2009;20[10]:1639–46.
12. Lehmann BD, Bauer JA, Chen X et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011 Jul;121[7]:2750-67.
13. Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 2004;4:423–36.
14. Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. The Journal of clinical investigation. 2000;105[8]:1045-7.
15. Browder T, Butterfield CE, Kräling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 2000;60[7]:1878–86.
16. Günther M, Wagner E, Ogris M. Acrolein: unwanted side product or contribution to antiangiogenic properties of metronomic cyclophosphamide therapy? J Cell Mol Med 2008;12:2704–16.
17. Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 2010;7[8]:455–65.
18. Nelius T, Klatte T, de Riese W, Haynes A, Filleur S. Clinical outcome of patients with docetaxel-resistant hormone-refractory prostate cancer treated with second-line cyclophosphamide-based metronomic chemotherapy. Med Oncol 2010;27:363–7.
19. C. Rochlitz, R. von Moos, M. Bigler, et al SAKK 24/09: Safety and tolerability of bevacizumab plus paclitaxel versus bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer—A multicenter, randomized phase III trial. J. Clin. Oncol. 32:5s, 2014 [suppl; abstr 518] 2014 ASCO Annual Meeting.
20. de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44[11]:1135-64.
21. Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res 2002;62:6938–43.
22. Colleoni M, Rocca A, Sandri MT et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002 Jan;13[1]:73-80.
23. Pietras K, Hanahan D. A multitargeted, metronomic, and maximumtolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. JClin Oncol 2005;23:939–52.
24. Sharabi A, Ghera NH. Breaking tolerance in a mouse model of multiple myeloma by chemoimmunotherapy. Adv Cancer Res 2010;107:1–37.
25. Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res 2009;69:4309–18.
26. Rabinovich GA, Rubinstein N, Matar P, Rozados V, Gervasoni S, Scharovsky GO. The antimetastatic effect of a single low dose of cyclophosphamide involves modulation of galectin-1 and Bcl-2 expression. Cancer Immunol Immunother 2002;50:597–603.
27. Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 2002;13:73–80.
28. Orlando L, Cardillo A, Rocca A, et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs 2006;17:961–7.
29. M. Stockler, T. Sourjina, P. Grimison, et al. A randomized trial of capecitabine [C] given intermittently [IC] rather than continuously [CC] compared to classical CMF as first line chemotherapy for advanced breast cancer. ASCO Annual Meeting Proceedings Part I. J. Clin. Oncol. 2007; 25[18S]:1031.
30. Fedele P, Marino A, Orlando L, et al. Efficacy and safety of low-dose metronomic chemotherapy with capecitabine in heavily pretreated patients with metastatic breast cancer. Eur J Cancer 2012 Jan;48[1]:24–9.
31. Addeo R, Sgambato A, Cennamo G, et al. Low-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancer. Clin Breast Cancer 2010;10[4]:301–6.
32. Miscoria M, Tonetto F, Deroma L, et al. Exploratory predictive and prognostic factors in advanced breast cancer treated with metronomic chemotherapy. Anticancer Drugs 2012;23:326–34.
33. Saridaki Z et al. A phase I trial of oral metronomic vinorelbine plus capecitabine in patients with metastatic breast cancer. Cancer Chemother Pharmacol 2012;69:35–42.
34. Cazzaniga ME, Torri V, Villa F, et al. Efficacy and safety of the all-oral schedule of metronomic vinorelbine and capecitabine in locally advanced or metastatic breast cancer patients: the phase I–II VICTOR-1 Study. Int J Breast Cancer 2014:769–90.
35. Hamano Y, Sugimoto H, Soubasakos MA, et al. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res 2004;64:1570–4.
36. Schwartzberg LS, Wang G, Somer BG, et al. Phase II Trial of Fulvestrant with metronomic capecitabine for postmenopausal women with hormone receptor-positive, HER2-Negative metastatic breast cancer. Clin Breast Cancer 2014:13–9.
37. Licchetta A, Correale P, Migali C, et al. Oral metronomic chemo-hormonaltherapy of metastatic breast cancer with cyclophosphamide and megestrol acetate. J Chemother 2010;22[3]:201–4.
38. Orlando L, Cardillo A, Ghisini R, et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer 2006;6:225.
39. García-Sáenz JA, Martín M, Calles A, et al. Bevacizumab in combiation with metronomic chemotherapy in patients with anthracycline- and taxanerefractory breast cancer. J Chemother 2008;20[5]:632–9. Dellapasqua S, Bertolini F, Bagnardi V, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 2008;26[30]:4899–905.
40. Montagna E, Cancello G, Bagnardi V, et al. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin Breast Cancer 2012;12[3]:207–14.
41. C. Rochlitz, R. von Moos, M. Bigler, et al SAKK 24/09: Safety and tolerability of bevacizumab plus paclitaxel versus bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer—A multicenter, randomized phase III trial. J. Clin. Oncol. 32:5s, 2014 [suppl; abstr 518] 2014 ASCO Annual Meeting.
42. Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer 2009;9[Suppl. 2]:S73–81.
43. Watanabe T, Sano M, Takashima S, et al. Oral uracil and tegafur compared with classic cyclophosphamide, methotrexate, fluorouracil as postoperative chemotherapy in patients with node-negative, high-risk breast cancer: National Surgical Adjuvant Study for Breast Cancer 01 Trial. J Clin Oncol 2009 Mar 20;27[9]:1368–74.
44. Crivellari D, Gray KP, Dellapasqua S, et al. Adjuvant pegylated liposomal doxorubicin for older women with endocrine nonresponsive breast cancer who are NOT suitable for a ‘‘standard chemotherapy regimen’’: the CASA randomized trial. Breast 2013:130–7.
45. Muss HB, Berry DA, Cirrincione CT et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med 2009; 360: 2055–2065.
46. Endo M, Shinbori N, Fukase Y et al. Induction of thymidine phosphorylase expression and enhancement of efficacy of capecitabine or 5'-deoxy-5-fluorouridine by cyclophosphamide in mammary tumor models. Int J Cancer. 1999 Sep 24;83[1]:127-34.
47. Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: final analysis of the randomized FinXX trial. J Clin Oncol. 2012 Jan 1;30[1]:11-8.
48. A Lluch, H Gomez, M Ruiz-Borrego et al. First Safety Data from a Randomised Phase III [CIBOMA 2004- 01/GEICAM 2003-11] Trial Assessing Adjuvant Capecitabine Maintenance Therapy after Standard Chemotherapy for Triple-Negative Early Breast Cancer. Cancer Res 2010;70[24 Suppl]:Abstract nr P5-10-15.
49. Alagizy HA, Shehata MA, Hashem TA, Abdelaziz KK, Swiha MM. Metronomic capecitabine as extended adjuvant chemotherapy in women with triple negative breast cancer. Hematol Oncol Stem Cell Ther. 2015 Mar;8[1]:22-7.
50. Shawky H, Galal S. Preliminary results of capecitabine metronomic chemotherapy in operable triple-negative breast cancer after standard adjuvant therapy--a single-arm phase II study. J Egypt Natl Canc Inst. 2014 Dec;26[4]:195-202.1
51. Colleoni M, Gray KP, Gelber S et al. Low-Dose Oral Cyclophosphamide and Methotrexate Maintenance For Hormone Receptor-Negative Early Breast Cancer: International Breast Cancer Study Group Trial 22-00. J Clin Oncol. 2016 Jun 20.