This paper describes the currently available drugs for the treatment of advanced kidney cancer. These are represented by more molecules that activate on vascular endothelial receptor (VEGFR): sunitinib, sorafenib, pazopanib, axitinib and VEGF inhibitors: bevacizumab. Another category is that of mTOR inhibitors: temsirolimus and everolimus. In Romania the following substances are available: sunitinib, sorafenib, temsirolimus and bevacizumab. In this context we present a typical case of metastatic clear cell renal cancer that received treatment with sunitinib (with stationary disease 9 months) and then after disease progression the patient received treatment with temsirolimus, the response being comparable with that obtained in trials published with this treatment.
Tratamentul carcinomului renal metastatic cu terapiile TKI şi mTOR: prezentare de caz
Treatment of metastatic renal cell carcinoma with TKI and mTOR therapy: a case report
First published: 24 martie 2015
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.30.1.2015.4310
Abstract
Rezumat
Lucrarea de faţă descrie medicamentele disponibile la ora actuală pentru tratamentul cancerului renal avansat. Acestea sunt reprezentate de mai multe molecule cu activitate asupra receptorilor vasculari endoteliali (VEGFR): sunitinib, sorafenib, pazopanib, axitinib şi a inhibitorilor de VEGF: bevacizumab. O altă categorie este aceea a inhibitorilor de mTOR: temsirolimus şi everolimus. În România sunt disponibile următoarele substanţe: sunitinib, sorafenib, temsirolimus şi bevacizumab. În acest context prezentăm un caz tipic de cancer renal cu celule clare metastatice care a beneficiat de tratament cu sunitinib (având boală staţionară 9 luni) şi ulterior după evoluţia bolii, pacientul a beneficiat de tratament cu temsirolimus, răspunsul fiind comparabil cu cel obţinut în trialurile publicate cu acest tratament.
Introduction
Renal cancer represents 4.3% of all adult neoplasiae according to Globocan estimation, representing the 7th cause of cancer in men and 9th cause of cancer in women. Annually, in the world are diagnosed 200,000 new cases of renal cancer and there are 100,000 deaths. This disease affects predominantly males in 2/1 rapport with women. Median age at diagnostic is 60 years.
In Romania renal cancer is situated on the 12th place of all cancers with an estimated incidence of 7.7/100,000.
Treatment schemas have suffered major changes in 2005/2007 when FDA and EMA approved a new series of molecules that target angiogenesis in advanced or metastatic renal cancer.
Since then, the number of new molecules registered in renal cancer treatment raised to 7. This fact is observed in survival benefit with an increased median survival from 26.4 months in 2006 to 32 months in 2014 as reported by JRMotzer in New England Journal in 2007 and at ASCO 2013.
First therapeutic classes used as antiangiogenic treatment were:
1. Anti VEGF - Bevacizumab - approved by EMA in December 2007 and by FDA in July 2009
2. Anti VGEFR:
a. Sunitinib, approved by EMA in August 2009 and by FDA in February 2007
b. Sorafenib, approved by EMA in December 2009 and by FDA in November 2007
c. Pazopanib, approved by EMA in July 2010 and by FDA in October 2009
d. Axitinib, approved by EMA in August 2012 and by FDA in January 2012.
3. MTor Inhibitors:
a. Temsirolimus approved by EMA in September 2009 and by FDA in May 2007
b. Everolimus approved by EMA in September 2009 and by FDA in May 2009.
Vascular endothelial growth factors (VEGFs) play a crucial role in tumour angiogenesis and development in renal cell carcinoma (RCC)(1,2). Advances in the understanding of RCC tumour biology have enabled the successful clinical development of molecular targeted therapies. Inhibition of angiogenesis by targeting the VEGF or mammalian target of rapamycin (mTOR) pathways has been shown to be an effective treatment for metastatic RCC (mRCC), improving overall response rates and progression-free survival (PFS) rates in mRCC compared with interferon (IFN)-based therapies(3).
In a Phase III clinical trial, first-line treatment with the VEGF receptor (VEGFR) tyrosine kinase inhibitor (TKI) sunitinib was shown to extend PFS (11 months vs 5 months) and lead to a higher response rate (31% vs 6%) compared with IFN-a(4).
In a Phase III trial, the mTOR inhibitor everolimus was shown to significantly extend PFS vs placebo (4.9 vs 1.9 months; p<0.0001) as second-line treatment in patients with mRCC who had progressed following treatment with sunitinib, sorafenib or both(5).
In a Phase III trial of second-line axitinib compared with sorafenib, PFS was 8.3 months (95% CI, 6.7-9.2) with axitinib vs 5.7 months (4.7-6.5) with sorafenib (HR, 0.656; 95% CI, 0.552-0.779; one-sided p<0.0001)(6).
The latest guidelines recommend sunitinib for first‑line therapy for mRCC.(7-9) Second-line therapies that are recommended after progression following treatment with a TKI include axitinib (TKI) and everolimus (mTOR inhibitor)(8).
Romania was among the first countries that reimbursed 4 targeted molecules in renal cancer: for patients with good and intermediary prognostic - Sunitinib, Sorafenib and Bevacizumab with Interferon and for patients with poor prognostic - Temsirolimus.
Since 2008 till present EMA and FDA approved other molecules for treatment, but Romanian reimbursement list was not updated, so treatments had to be done with those molecules.
Here we present a case of a patient with metastatic clear cell RCC with a treatment effect following sequential VEGF and mTOR inhibitor treatment due to accessibility to mTOR inhibitor.
Patient presentation
January 2011: A 50-year-old man, with no previous relevant personal or family medical history, presented with right lumbar pain, which was resistant to analgesia.
February 2011:
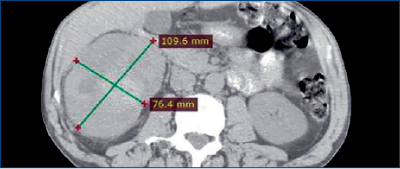
- Abdominal CT scan revealed a mass in the right kidney (109 x 76 mm) (Figure 1) and thoracic CT scans showed no metastases.
- The patient consequently underwent a radical nephrectomy. Subsequent histology revealed this was a clear cell RCC (pT3apN0), with peri-renal fat extension
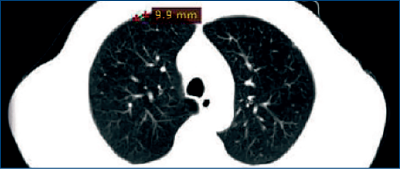
January 2012: A routine CT scan revealed pulmonary metastases (Figure 2).
March 2012: The patient began sunitinib (50 mg PO QD, 4 weeks on/2 weeks off) after obtaining approval of Romanian Insurance System.
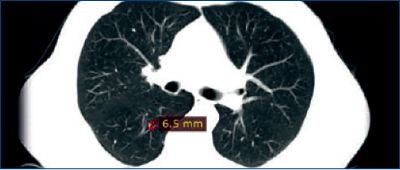
September 2012: No change in the size of the pulmonary metastases was observed on routine CT scans
- The patient was deemed to have stable disease (RECIST 1.1).
- The patient did not experience any side effects with sunitinib treatment.
January 2013:
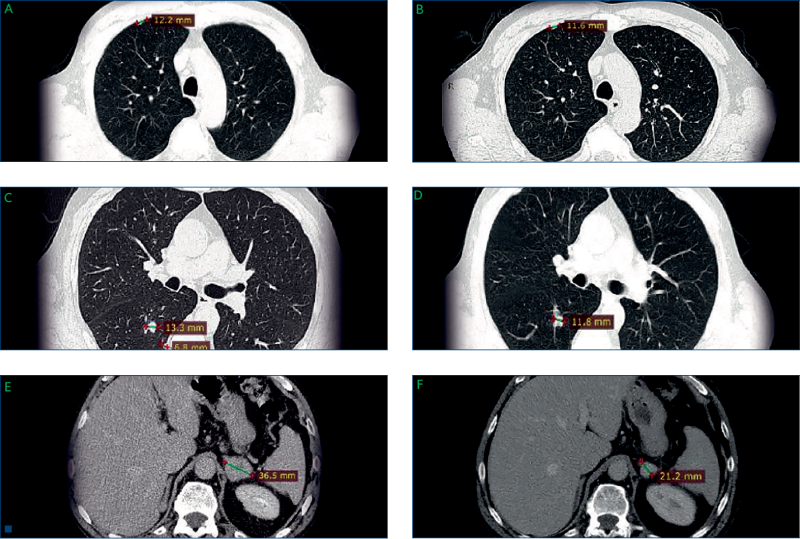
- Routine CT demonstrated that the pulmonary metastases had increased in size (Figures 3A and C), and a further metastasis was observed in the left adrenal gland (Figure 3E).
- Sunitinib treatment was stopped after six cycles, and second-line treatment with temsirolimus was prescribed*.
February 2013: The patient began temsirolimus (25 mg IV weekly) treatment.
May 2014:
- Temsirolimus treatment is ongoing and the patient has not experienced any side effects.
- Routine CT scan revealed shrinkage of the lung and the adrenal metastases (Figure 3B and D, and Figure 3F, respectively), and the patient was considered to have a partial response (by RECIST criteria).
Conclusions
Here we supply case details of a patient with mRCC treated with sunitinib, who had a PFS of approximately 9 months, similar to the PFS observed in clinical trials(4). Sunitinib was also well tolerated by this patient.
Temsirolimus, an mTOR inhibitor, is currently only approved for the first-line treatment of mRCC patients with poor prognosis(10).
Here we demonstrate a treatment effect of second‑line temsirolimus in a patient with mRCC, which suggests that this may be an option for treatment if everolimus is unavailable.
Bibliografie
2. Tsuchiya N, et al. Quantitative analysis of gene expressions of vascular endothelial growth factor-related factors and their receptors in renal cell carcinoma. J Exp Med. 2001;195:101–113.
3. Escudier B, et al. Treatment selection in metastatic renal cell carcinoma: Expert consensus. Nat Rev Clin Oncol. 2012; 9:327–337.
4. Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124.
5. Motzer RJ, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer. 2010; 116:4256–4265.
6. Motzer RJ, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562.
7. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Kidney Cancer. Available from: http://www.nccn.org/ professionals/physician_gls/pdf/kidney.pdf. Last accessed August 2014.
8. Escudier B, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl. 3):iii49–iii56.
9. Ljunberg B, et al. EAU Guidelines: Guidelines on Renal Cell Carcinoma. Available from: http://www.uroweb.org/gls/pdf/10%20Renal%20Cell%20Carcinoma_LR.pdf. Last accessed August 2014.
10. Temsirolimus SmPC/PI. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000799/WC500039912.pdf. Last accessed October 2014.
Articole din ediţiile anterioare
New approaches to colorectal cancer
Cancerul colonului reprezintă una din cele mai frecvente localizări ale cancerului în epoca noastră.
Muscle cramps in cancer patients – one reason to jump off the bed
Crampele musculare reprezintă o simptomatologie destul de frecvent întâlnită la mulţi dintre pacienţii cu boli oncologice. Cauzalitatea acestu...
Îngrijirile paliative – prezent şi viitor
Îngrijirile paliative iau o amploare din ce în ce mai mare în întreaga lume, iar pandemia de COVID-19 a accentuat nevoia pentru astfel de îngrijiri.