NETs are rare diseases.
There is a lack of robust source data on epidemiology
Nothing exists to describe the overall European situation:
-
Only individual country data exists
-
ENETS in process of establishing a pan-European registry.
Rare locations:
-
Liver and biliary system (0.59%)
-
Gall bladder (0.31%)
-
Meckel’s diverticulum (0.27%)
-
Oesophagus (0.2%)
Lack of awareness frequently leads to misdiagnosis or no diagnosis
-
Higher incidence in African-American than Caucasian patients
-
Potential genetic factors influencing this are currently unknown.
Rectal NETs are more common in Asian/Pacific Islander, American Indian/Alaskan Native and African American patients(4).
Race predicts outcome in patients with well-differentiated to moderately differentiated NETs (P<0.001)(4).
Tumor types and clasification
NENs -malignant tumours that arise from the diffuse neuroendocrine cell system.
May or may not show hypersecretion of peptides or amines causing hormonal symptoms.
Functional vs non-functional
The term NENs= the whole family of low, intermediate and high grade tumours:
-
NETs - low to intermediate grade neoplasms
-
NEC - only be used for high grade neoplasms.
Categorised according to their embryonic origin:
-
Foregut
-
Midgut
-
Hindgut.
Biologically relevant differences in tumours not distinguished:
Current practice describe GEP-NETS according to their location of primary origin:
-
e.g., pancreas, duodenum, small intestine etc.
-
e.g., gastrinoma, insulinoma, carcinoid syndrome etc.
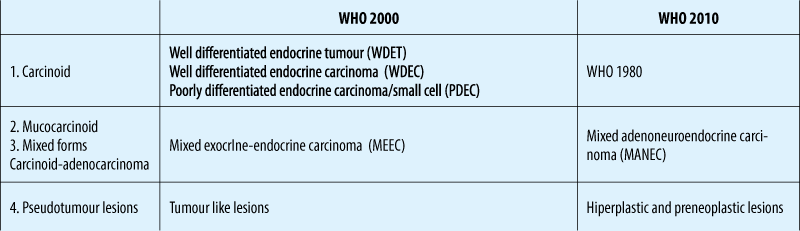
WHO clasification
TNM staging (ENETS)
Grading (ENETS)
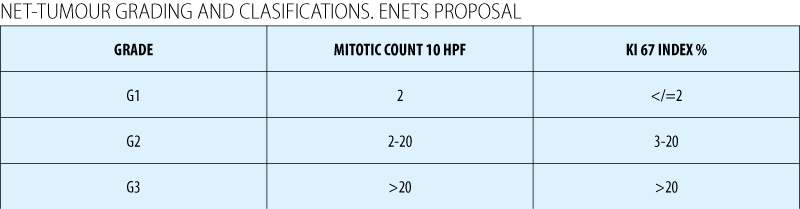
Assessment of mitotic rate: Ki67
In Europe - Ki67 is mandatory for all cases (ENETS and WHO 2010)(1)
Difficulties when:
-
There is insufficient biopsy material to differentiate between Grade 1 and 2 NETs
-
When a large amount of crush artefact present.
Cell cycle-dependent marker found in higher concentrations in dividing cells.
Immunohistochemistry methods:
-
An antibody called MIB1 can reveal Ki67, indicating the level of proliferation.
A high Ki67 index indicates a fast-growing tumour - the worse the prognosis.
Diagnosis
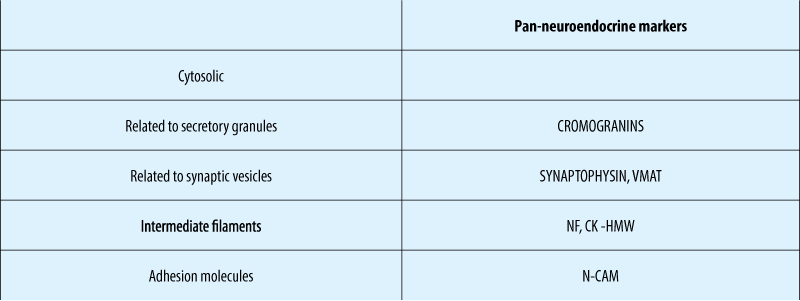
Immunohistochemical NE – markers
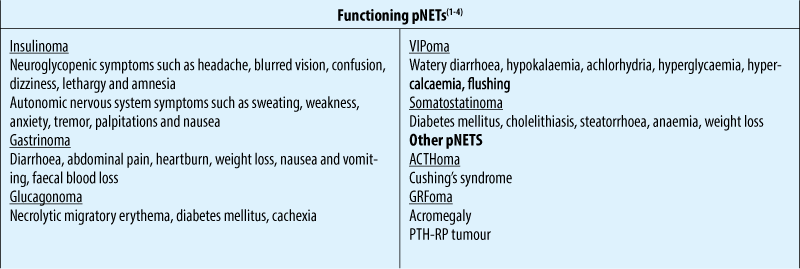
Non-functional pNETs
-
Can secrete pancreatic polypeptide, chromogranin A, neuron specific enolase, human chorionic gonadotrophin subunits, calcitonin, neurotensin or other peptides
-
Do not produce specific symptoms.
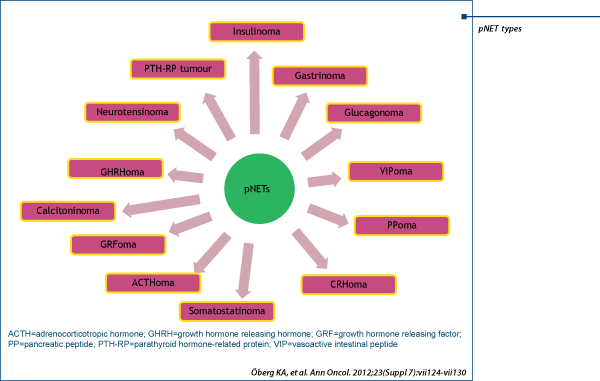
Functional pNETs
-
Are named based on the specific hormone they produce (i.e. insulin, gastrin, somatostatin, glucagon etc.)
-
Most common are insulinoma and gastrinoma (Zollinger-Ellison syndrome [ZES])
-
Other types of functional pNETs are grouped as rare functional pNETs (RFTs)
Chromogranin A (Cga)
Chromogranins A and B are protein precursors involved in the regulation of hormone secretion(1).
CgA is expressed in well-differentiated tumours(2). Can be expressed in less well-differentiated tumours that do not secrete known hormones(3).
NSE - in G3
Common conditions can increase the levels of CGA
-
Decreased renal function
-
Treatment with Proton Pump Inhibitors (PPIs)
-
Chronic gastritis
-
Essential hypertension.
Measurement of CgB as a complement to CgA has been suggested(1,2).
CgA blood levels vary according to tumour characteristics
-
Tumour mass -small tumours may be associated with normal CgA levels
-
Tumour burden
-
Progression and malignant nature of the tumour.
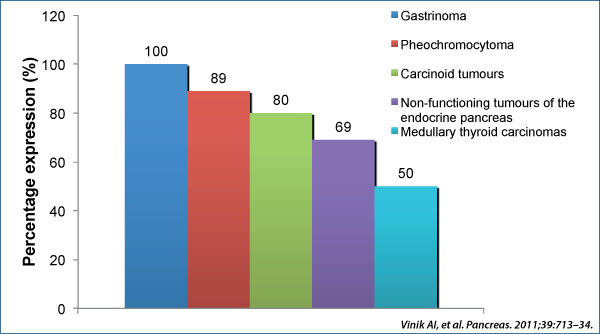
Many NETs of non-pancreatic origin release vasoactive peptides and amines into the systemic circulation and cause a characteristic set of symptoms called “carcinoid syndrome”(1):
-
e.g., serotonin and tachykinins.
It occurs in approximately 10% of patients with metastatic NETs(1)
Characterised in patients by:
-
Flushing (63-94%)
-
Diarrhoea (68-84%)
-
Abdominal pain (10-55%)
-
Telangiectasia (25%)
-
Bronchoconstriction (3-19%)
-
Carcinoid heart disease.
NETS FUNCTIONING pNETS - SyMpTOMS AND SYNDROMES
Treatment options
Management of locoregional unresectable or/and metastatic disease ases
Total tumor eradication
If total eradication not possible, then:
-
Symptomatic control
-
Prevention of complications related to the carcinoid syndrome( carcinoid heart disease, carcinoid crisis)
-
Inhibition of tumor growth/prolongation of survival.
Surgical Approaches - curative ablative
Debulking procedures
-
TACE, TAE, RFA, SIRT etc.
Medical therapy.
-
Biotherapy
·somatostatin analogues ( octreotide, lanreotide, pasireotide)
·a IFN.
-
Systemic chemotherapy
-
Molecular targeted therapies
·angiogenesis inhibitors: sunitinib, sorafenib, bevacizumab
·mTOR inhibitors: everolimus
·GF- rec inhibitors: EGF-R TKI etc.
Peptide Receptor Radionuclide Therapy (PRRT).

NCCN guidelines for metastatic NET
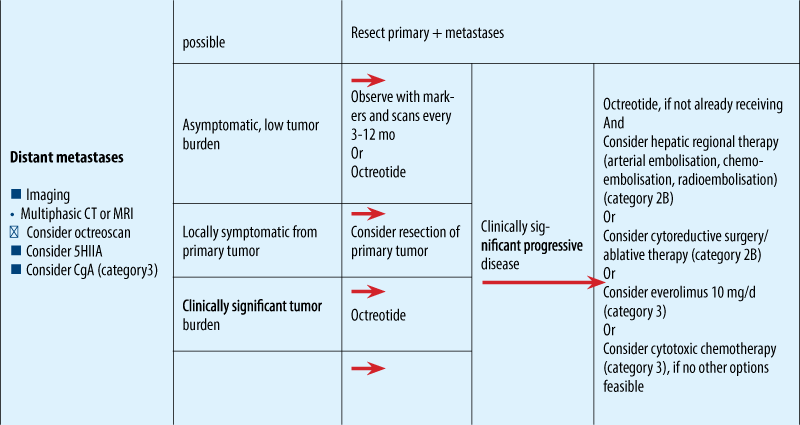
ESMO clinical guidelines
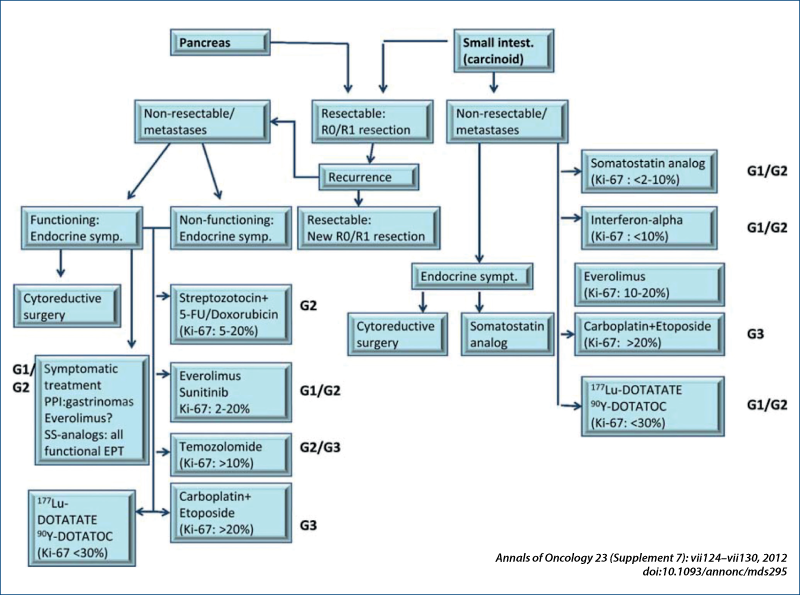
Treatment approach to liver MTS without ENETS Consensus Guidelines for LIVER MTS

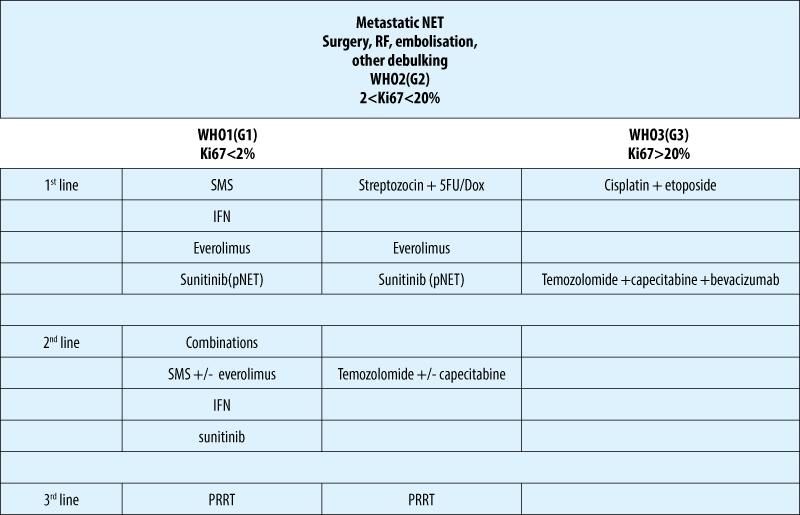
Treatment algoritm NET - based on classification
Biotherapy - somatostatin analogues
-
the use of SSA is the standard therapy in functioning NETs of any site
-
octreotide and lanreotide are considered equally effective for syndrome control (70-90% of cases)
-
a standard dose of long-acting formulations is octreotide 20-30 mg/4 weeks i.m. and lanreotide autogel 90-120mg/4 weeks s.c.
-
doses are adapted to the individual needs and depend on tumor burden
-
preventive SSA therapy prior to surgery or use of locoregional therapies ( s.c. bolus and/or i.v. 50-100 µg/h perfusion) is usually effective.
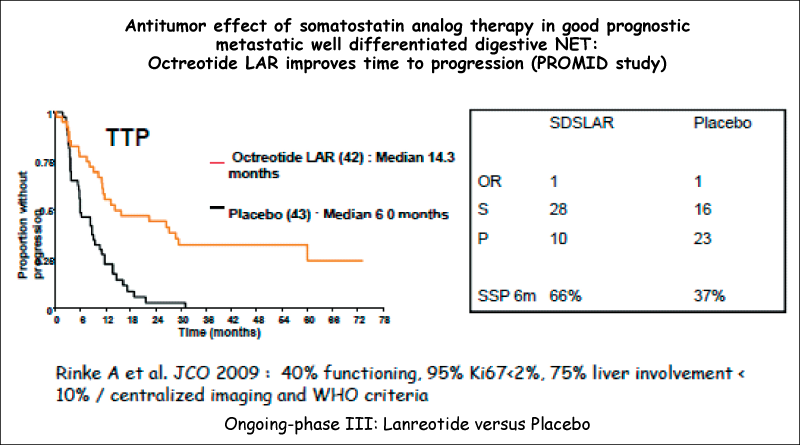
Significant improvement in TTP regardless of the presence of carcinoid syndrome
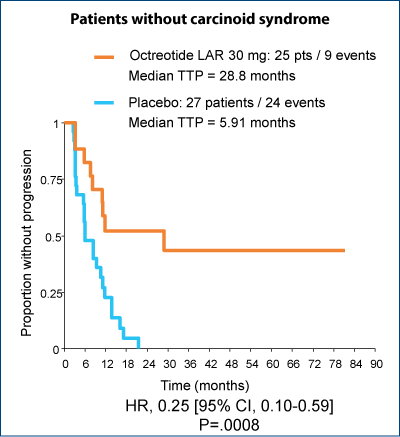
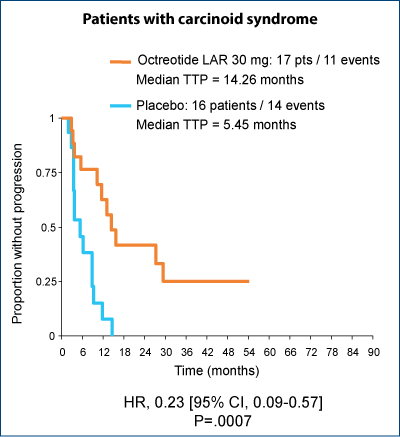
Biotherapy - IFN
-
may also be considered for symptom control, if SSA are not well tolerated
-
symptomatic remission - 30-70%
-
stabilisation/remission of tumor markers - 40%
-
tumor PR/SD - 10%
-
onset of response is more delayed than with SSA
-
recommended dose of 3-5 milion units 3 times per week
-
pegylated IFN may be considered for better tolerability ( 80-150 µg once weekly).
CHEMOTHERAPY
Few data to support the use of any existing cytotoxic chemotherapy agents.
Result are poor in patients with well-differentiated tumors, with RR of 15% in the largest published study
Option exclusively in advanced intestinal NET after failure to previous treatment lines.
Chemotherapy is the first-line therapy in NEC G3.
No clear cut-off value for Ki67 for indication of chemotherapy.
In cases of liver MTS from NEC G3, regardless of the site of the primary tumor combination chemotherapy - CISPLATIN + ETOPOSIDE is recommended early.
There is no established second line for G3 NEC
-
Temozolomide + capecitabine +/- bevacizumab;
-
CAPOX/FOLFOX/FOLFIRI.
Molecular targeted therapies
Angiogenesis inhibitors: sunitinib, sorafenib, bevacizumab.
mTOR inhibitors: everolimus.
GF–rec inhibitors: EGF-R TKI etc.
RADIANT-2 study design
Phase III double-blind placebo-controlled trial
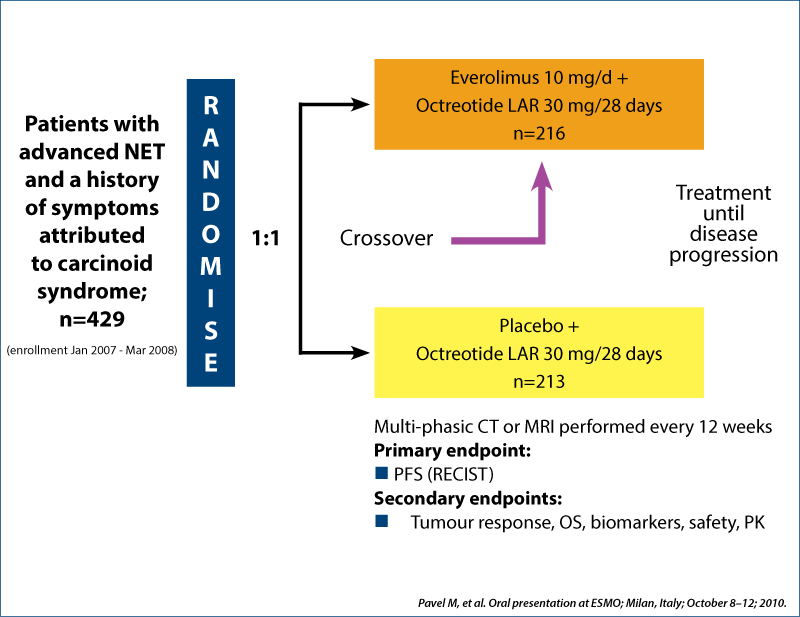
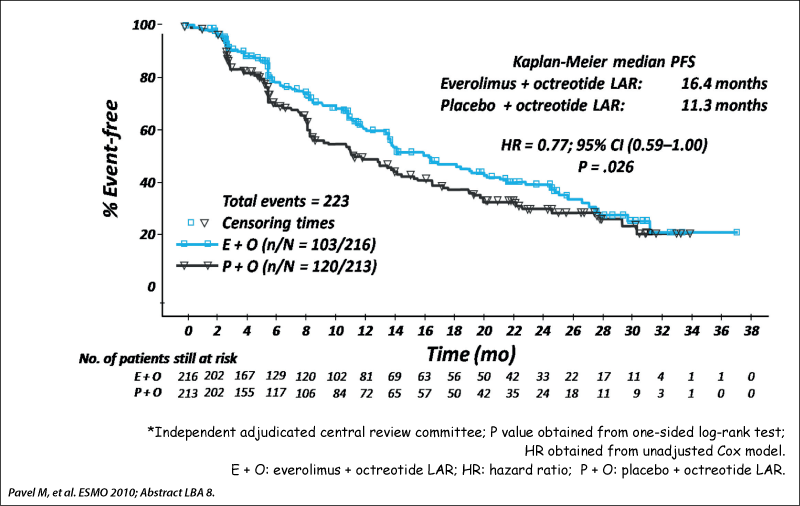
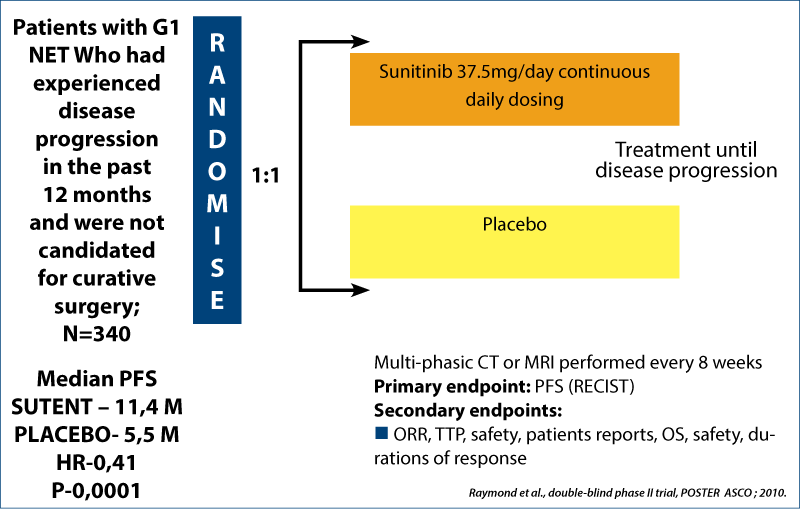
Sunitininb in net
Phase III double-blind placebo-controlled trial
Pazonet study - pazopanib in pretreated advanced neuroendocrine tumors - a phase II, open-label trial of the Spanish task Foirce Group for NETs
Results
44 patients
Prior treatment- multitarget therapy( sunitinib)
-
mTOR inhibitors
-
both agents
Treatment- pazopanib 800 mg/zi, 28 days, +/- SSA
25 patients - was progression free at 6 m/mPFS - 9.5 months
21 patients -SD
· 73% for patients treated with multitarget inhibitor.
· 60% for patients treated with mTOR inhibitor.
· 25% -for patients treated with both agents.
Evaluated pazopanib as single agent in advanced NETs after failure of the other systemic treatments
-
Pazopanib - multitargeted for:
·Vascular endothelial growth factor receptor 1, 2, 3 VEGFR
·Platelet derived growth factor receptor alfa and beta PDGFR
· Proto-oncogene c Kit.
Primary end point - CLINICAL BENEFIT RATE (CR +PR+SD) at 6 months
-
Was evaluated translational corelation of radiology response and PFS with circulating and tissue biomarkers.
Results - biomarkers
Non-significant increase of PFS was observed in patients presenting:
lower baseline circulating tumor cell
decreased levels of soluble VEGFR 2
VEGFR 3 GENE polimorphisms.
(Potential biomarkers for selecting patients for pazopanib)
![177Lu-DOTATATE:177Lu-1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid0 (DOTA), Tyr3-octreotate; 90Y DOTATOC: [90Y-DOTA]-D-Phe1-Tyr3-octreotide. 1. Kwekkeboom DJ et al. J Clin Oncol. 2008;26:2124-2130. 2. Waldherr C et al. Ann Oncol. 2001;12:941-944.](/image/15036/0/asset_1_15036.png)
Peptide Receptor Radiotherapy (PRRT)
Systemic radiotherapy targeting somatostatin receptors
Compounds vary by isotope and carrier molecule
177Lu DOTATATE(1) and 90Y DOTATOC(2) more frequently used
RR (0-37%) is higher in pNET compared to midgut NET.
The use of PRRT is after failing the first line medical therapy.
The presence of expression of SSRT2 is a prerequisite for the use of PRRT.
A better effect is reported with increasing SSTR expression according to SRS.
Serious side effects: severe bone marrow disease, kidney failure, liver failure.
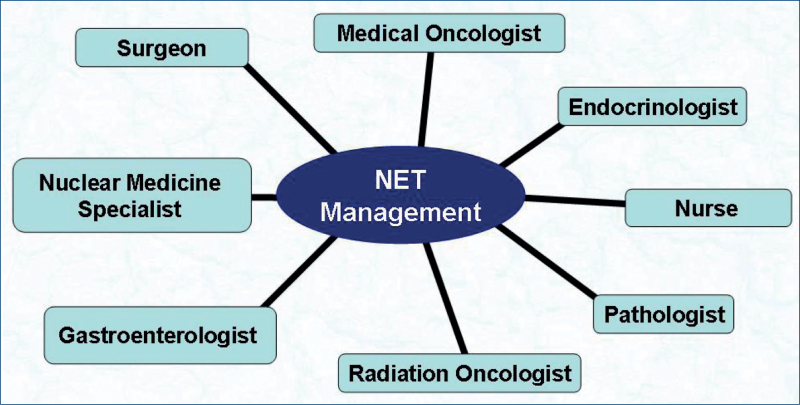
Multidisciplinary approach in the management of NETs