Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, originating in the uveal tract of the ocular globe. Spontaneous necrosis of choroidal melanomas is very rare, but some UMs may be predisposed to spontaneous necrosis due to rapid growth. The diagnosis of an extensively necrotic uveal melanoma can be challenging, because the associated intraocular hemorrhage makes impossible the direct tumor visualization during ophthalmoscopy. Ultrasonography is an imagistic tool based on sending and receiving sound waves (frequency above 20,000 Hz) with a probe which contains a piezoelectric transducer and works like a transmitter and receiver of sound waves in the same time. Ocular ultrasonography is the most commonly used imaging technique for determining the presence of a uveal melanoma, especially in opaque ocular media when dilated fundus examination of the eye cannot be made. In ophthalmology, we use two types of sound waves: an A-mode scan which uses frequencies of 8 MHz, and a B-mode scan for the visualization of intraocular structures which uses frequencies of 10 MHz. Ocular ultrasonography has many advantages in clinical practice, due to availability, no exposure to radiation and to low price. This article presents the difficulties associated with the diagnosis of necrotic uveal melanoma in the absence of fundus eye examination due to ocular opaque media. In this case, we should do the differential diagnosis with other pathologies that could resemble echographically uveal melanoma. In cases when ultrasonography cannot establish a clear diagnosis, we should use ancillary test.
Ultrasonography in necrotic choroidal melanoma
Ultrasonografia în melanomul coroidal necrotic
First published: 26 octombrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.64.3.2023.8778
Abstract
Rezumat
Melanomul uveal este cea mai frecventă tumoră malignă intraoculară la adulţi, cu originea în tractul uveal al globului ocular. Necroza spontană a melanoamelor coroidale este foarte rară, dar unele melanoame uveale pot fi predispuse la necroză spontană, din cauza creşterii rapide. Diagnosticul unui melanom uveal extensiv necrotic poate fi o provocare, deoarece hemoragia intraoculară asociată face imposibilă vizualizarea directă a tumorii în timpul oftalmoscopiei. Ultrasonografia oculară este un instrument imagistic bazat pe trimiterea şi receptarea undelor sonore (cu frecvenţă de peste 20.000 Hz), utilizând o sondă ce conţine un traductor piezoelectric şi care funcţionează ca un emiţător şi receptor de unde sonore în acelaşi timp. Ecografia oculară este tehnica imagistică utilizată cel mai frecvent pentru determinarea prezenţei unui melanom uveal, în special în mediile oculare opace, când examinarea fundului dilatat al ochiului nu poate fi realizată. În oftalmologie folosim două tipuri de unde: o scanare în modul A, care utilizează frecvenţe de 8 MHz, şi o scanare în modul B, pentru vizualizarea intraoculară a unor structuri, care utilizează frecvenţe de 10 MHz. Ecografia oculară are multe avantaje în practica clinică, datorită disponibilităţii, fără expunere la radiaţii şi cu un cost redus. Acest articol prezintă dificultăţile asociate cu diagnosticul de melanom uveal necrotic, în absenţa examinării fundului de ochi, din cauza mediilor oculare opace. În acest caz, ar trebui să facem diagnosticul diferenţial cu alte patologii ce ar putea semăna ecografic cu melanomul uveal. În cazurile în care ultrasonografia nu poate stabili un diagnostic clar, se pot folosi şi alte teste.
Introduction
Uveal melanoma (UM) is a neoplasm that originates from melanocytes(1). In 90% of cases, it develops from the choroid, in 7% of cases from the ciliary body, and in 3% from the iris(2-4). In the anterior, the tumor usually localizes in the uveal tract containing the iris. In the posterior, the tumor may localize on the choroid or ciliary body(5).
The diagnosis of uveal melanoma is based primarily on clinical examination by biomicroscopy and indirect ophthalmoscopy(6). In contrast to the basic principles of oncology, histological or cytologic evaluation is not routinely used in the diagnosis of intraocular neoplastic lesions(6).
Ancillary tests, including color fundus photography, ultrasonography (USG), fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), optical coherence tomography (OCT), fundus autofluorescence (FAF) and ultrasound biomicroscopy (UBM), can be used in order to confirm the diagnosis(6).
Fine-needle aspiration biopsy (FNAB) of the tumor can be performed when the clinical diagnosis is unclear, and the diagnosis can be clarified by the evaluation of an experienced ocular pathologist(6).
Ophthalmic ultrasonography uses high-frequency sound waves which are transmitted from a probe into the eye(7).
As sound waves strike intraocular structures, they are reflected back to the probe and are converted into an electrical signal(7).
This signal is subsequently reconstructed as an image on a monitor that can be used to perform a dynamic evaluation of the eye or photographed to document the pathology(7).
Sound is emitted in a parallel, longitudinal wave pattern. The frequency of the sound wave is the number of cycles, or oscillations, per second, measured in hertz (Hz). For sound to be considered ultrasound, it must have a frequency higher than 20,000 oscillations per second, or 20 KHz, rendering it inaudible to human ears(3). The higher the frequency of the ultrasound, the shorter the wavelength (the distance from the peak of one wave to the peak of the next wave). A direct relationship exists between the wavelength and the depth of tissue penetration (the shorter the wavelength, the more shallow the penetration). However, as the wavelength shortens, image resolution improves(7).
Given that ophthalmic examinations require little in the way of tissue penetration (with an eye measuring 23.5 mm long on average) and much in the way of tissue resolution, ultrasound probes used for ophthalmic B-scan are manufactured with very high frequencies of about 10 million oscillations per second, or 10 MHz. In contrast, ultrasound probes used for purposes such as obstetrics use lower frequencies for deeper penetration into the body, and because the structures being imaged are larger, they do not require the same degree of resolution. High-resolution ophthalmic B-scan probes – ultrasound biomicroscopy (UBM) – of 20 to 50 MHz that penetrate only about 5 to 10 mm into the eye for incredibly detailed resolution of the anterior segment have been manufactured(8).
Ultrasound has been extensively used as an effective diagnostic tool in the evaluation of uveal melanoma since the 1950s, when A-mode ultrasound was first applied(9).
In general, most uveal melanomas are dome or mushroom shaped, and each dome or mushroom exhibits a unique growth progression. They may appear biconvex, spheroid, ellipsoid or hemiellipsoid during their growth(10-12) and eventually become irregular as they break through the Bruch’s membrane.
Although apical height and basal diameter measurements are used for monitoring tumor growth or regression, 3D volume, which is independent of tumor shape, offers an objective estimate of the tumor size and may be important in the evaluation of melanoma progression(13-15).
In ophthalmology, two types of ultrasonography are used: an A-mode scan to carry out measurements or a movability assessment, and a B-mode scan to visualize intraocular structures of different echogenicities as a grayscale view created, based on reflected waves(16).
These modes use different frequencies: the A-mode uses 8 MHz and the B-mode uses 10 MHz(16,17).
A-scan ultrasonography of the eye is useful for tumors thicker than 2-3 mm.
Uveal melanoma characteristically shows an initial prominent spike, followed by low-to-medium internal reflectivity with diminishing amplitude and a significant echo. Vascular pulsations can be seen as fine oscillations of the internal spiking pattern within the tumor. Standardized ultrasonography has a diagnostic accuracy above 95%. Performing sequential A-scans, with accurate dimension measurements, in cases of diagnostic uncertainty, is important(18).
B-scan ultrasonography of the eye is a routine test used in the evaluation of any posterior segment mass. It is especially needed in patients with media opacity. For choroidal melanomas, B-scan ultrasonography is used to help establish the diagnosis, to evaluate possible extraocular extension, to estimate tumor size for periodic observation, and to plan therapeutic intervention(18).
Intraocular melanomas have several distinctive ultrasonographic features, as follows:
-
Low-to-medium reflectivity
-
Excavation of underlying uveal tissue
-
Shadowing of subjacent soft tissues
-
Internal vascularity
-
An acoustic quiet zone at the base of the tumor called acoustic hollowing(18).
Uveal melanoma on an A-scan has low to medium reflectivity and presents a positive angle kappa sign, which means that the spikes are high and decrease toward the sclera(1). This kappa sign is a very important tool to establish an accurate ultrasonographic diagnosis of uveal melanoma.
The A-scan-based methodology developed by Ossoinig was utilized in the Collaborative Ocular Melanoma Study (COMS) – the largest study performed in ocular oncology – and it has become a standard for the ultrasound diagnosis of choroidal melanoma(19-21).
Ultrasonography is commonly used in clinical practice in the diagnosis of uveal melanoma due to availability, no exposure to radiation, and to low price(16).
Vascularity of uveal melanoma may be more difficult to see on diagnostic A-scan exams, but many times it is easy to see on the B-scan exam. Vascularity is appreciated as areas of pulsation within the lesion(22).
Necrotic choroidal melanoma
Spontaneous necrosis of uveal melanomas is very rare, varying between 0.5% and 1% of all uveal melanomas(23).
The incidence of totally necrotic uveal melanomas has been reported at 3.6% and that of partially necrotic UMs as 5.7% in previous studies(24). These tumors are usually associated with significant hemorrhage and/or inflammation(23,25).
The mechanism of infarction and necrosis of uveal melanomas and ocular structures is not fully understood(26).
Brannan et al.(27) proposed two potential mechanisms of infarction with associated ocular necrosis. One mechanism that has been discussed involves the concept of “watershed infarction” of UMs typically located at the equator(26).
The choroid is supplied by the short posterior ciliary artery, the iris and the ciliary body by the long posterior ciliary artery via anastomosis at the ciliary plexus with the anterior ciliary artery (all branches being fed by the ophthalmic artery). “Watershed” infarction of a uveal melanoma requires a vascular compromise from both the anterior and posterior circulation of the eye(26).
The complete infarction of UMs observed in previous studies is likely to have progressed from watershed infarctions that spread to include the entire tumors as ischemia continued(26).
If any intact tumor cells were found in the necrotic melanoma, they tended to be located at the tumor periphery, thus supporting the concept of watershed ischemic necrosis in the center of the tumor with outward expansion of ischemic necrosis(26).
This mechanism is further supported by a lack of thrombi in the long posterior ciliary arteries, short posterior ciliary arteries, and the major circle of the iris in all their specimens(23,27).
The second mechanism proposed for infarction in uveal melanoma is that the tumor mass of the UM raises the intraocular pressure, leading to acute angle closure, vascular compromise, and ischemia of the ocular contents(27).
It has been also stipulated that a uveal melanoma grows too rapidly, outstrips its blood supply and becomes necrotic. The tumor growth leads to the release of cytotoxic products triggered by the necrosis, which leads to vasculitis of intraocular vessels and, consequently, to infarction, swelling and cellulitis of ocular and extraocular tissues(26).
This would represent the spontaneous version of toxic tumor syndrome, which is induced via the proton beam when administered to large tumors(28).
Vasculitis provides a potential explanation for the infarction of both the anterior and posterior choroid which typically avoids the vascular compromise due to vessel anastomoses(27,29).
Sudden infarction could then lead to the acute pain and inflammation of the sclera and episcleral, often experienced by the patients.
Materials and method
After obtaining the ethics approval from the Clinical Emergency Eye Hospital Bucharest, we performed ultrasound investigations on two patients who presented opaque ocular media, and we could not perform the clinical examination of the posterior segment of the eye. To perform the ultrasound examinations, we used the Quantel ABSolu echograph produced by Quantel Medical, France.
Case 1. A 62-year-old Caucasian female was referred to the Clinical Emergency Eye Hospital Bucharest for progressive visual loss of six-month duration in the right eye (OD), with no pain or inflammation. Anterior segment examination in the right eye revealed a mature cataract with a vision of light perception, intraocular pressure of 16 mmHg, and with no view of the posterior pole because of the dense cataract. The ocular findings of her left eye (OS) were normal. She did not have any significant past ocular history or family history of ocular disease or cancer, diabetes or high blood pressure, and she was a nonsmoker.
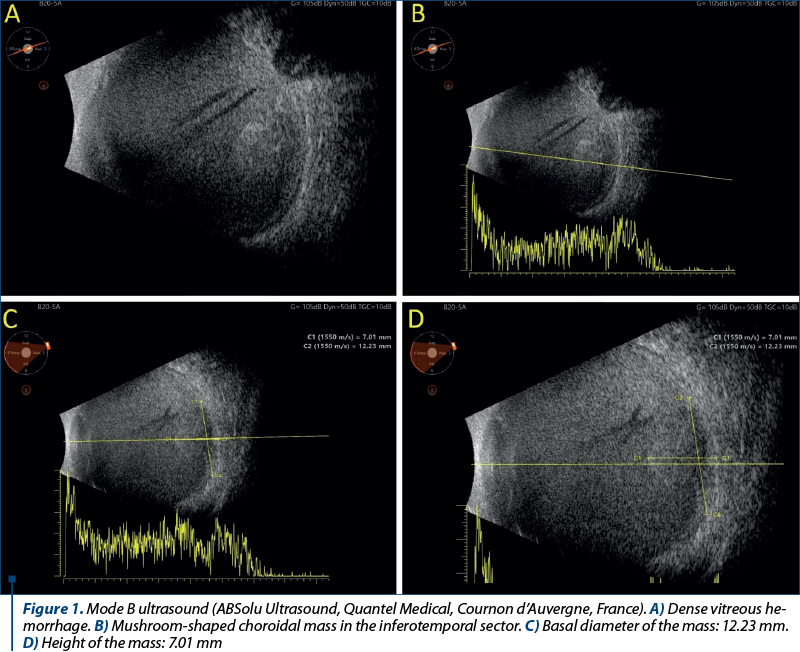
On the ultrasonographic examination of the right eye, we found multiple punctate vitreous echoes, medium and high reflectivity, mobile, suggestive for a vitreous hemorrhage. At a careful look, we discovered in the inferonasal sector a dome-shaped choroidal mass with an anteroposterior diameter of approximately 12.11 mm and with choroidal excavation present, suggestive for a choroidal melanoma.
In Figure 1, we have four images in B-mode USG which are showing dense vitreous hemorrhage, and in the inferotemporal sector, the mushroom-shaped choroidal mass.
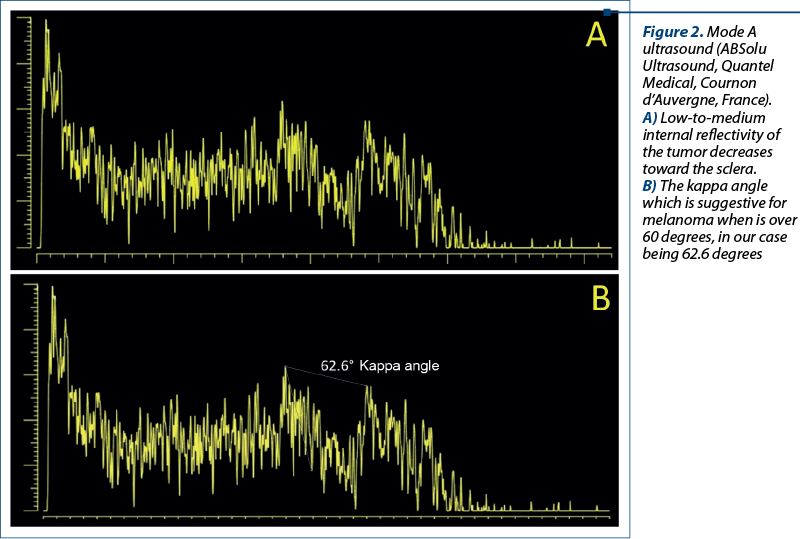
In Figure 2, we have two images in A-mode USG which are showing the low-to-medium internal reflectivity of the tumor decreases toward the sclera. In the second picture, we could calculate the kappa angle which is suggestive for melanoma when is over 60 degrees, in our case being 63.2 degrees.
Case 2. A 67-year-old Caucasian female was referred to the Clinical Emergency Eye Hospital Bucharest for progressive visual loss of two-month duration in the right eye (OD), with pain and inflammation. The anterior segment examination in the right eye revealed conjunctival and episcleral vessels dilated and congested with a vision of light perception, intraocular pressure of 22 mmHg, and with no view of the posterior pole because of a dense vitreous hemorrhage. The ocular findings of her left eye (OS) were normal. She did not have any significant past ocular history or family history of ocular disease or cancer, diabetes or high blood pressure, and she was a nonsmoker.
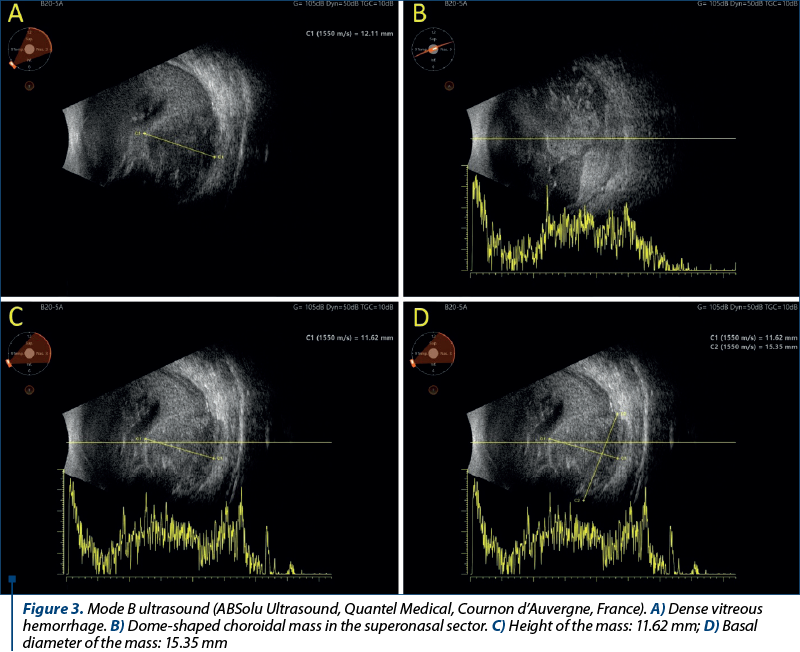
On ultrasonographic examination of the right eye, we found multiple punctate vitreous echoes, medium and high reflectivity, mobile, suggestive for a vitreous hemorrhage. At a careful look, we discovered in the inferotemporal sector a mushroom-shaped choroidal mass with an anteroposterior diameter of approximately 12.23 mm and choroidal excavation present suggestive for a choroidal melanoma.
In Figure 3, we have four images in B-mode USG which are showing dense vitreous hemorrhage, and in the superonasal sector, the dome-shaped choroidal mass.
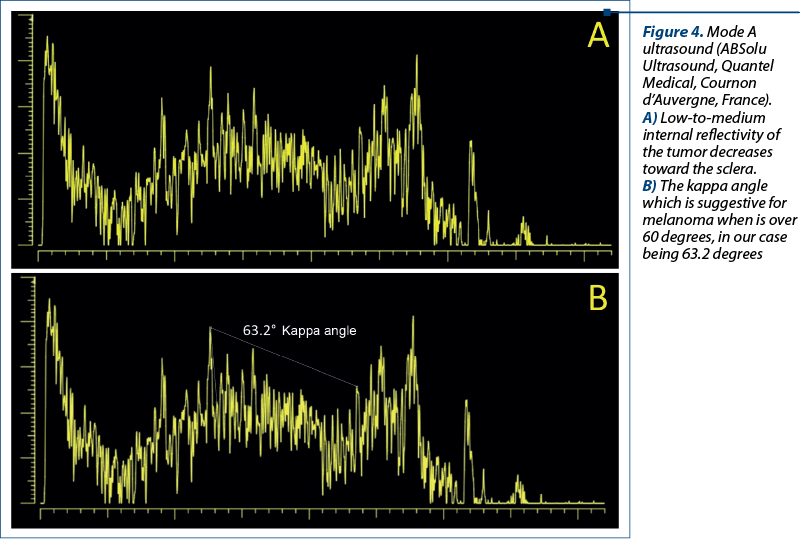
In Figure 4, we have two images in A-mode USG which are showing the low-to-medium internal reflectivity of the tumor decreasing toward the sclera. We could calculate kappa angle which is suggestive for melanoma when is over 60 degrees, in our case being 63.2 degrees.
Discussion
Spontaneous necrosis of choroidal melanomas is very rare, but some uveal melanomas may be predisposed to spontaneous necrosis due to rapid growth. The diagnosis of an extensively necrotic uveal melanoma can be challenging, because the associated intraocular hemorrhage makes impossible the direct tumor visualization during ophthalmoscopy.
In addition to the lack of tumor visualization, the results of ancillary testing in eyes with necrotic uveal melanoma can be atypical, adding to the diagnostic difficulties(30).
Eye transillumination in the setting of severe intraocular hemorrhage shows diffusely dark choroid with no identifiable tumor shadow(30).
On ultrasonography, the dense echoes resulting from subretinal or suprachoroidal hemorrhage can obscure even a large underlying ciliary body or choroidal tumor(30).
The detection of spontaneous vascular pulsations on ultrasonography is an important feature in distinguishing a posterior uveal melanoma from hemorrhage, but vascular pulsations can be absent in the setting of extensive tumor necrosis(31).
The characteristic MRI T1 hyperintensity of choroidal melanoma can be obscured in the presence of a T1 hyperintense subacute hemorrhage(30).
In such cases, the tumor may be best visualized on T2-weighted MRI, where melanoma is hypointense and can be better distinguished from evolving hemorrhage(32).
Uveal melanomas show enhancement with gadolinium, but as the current case shows, this feature can be minimal or absent in necrotic melanoma(33).
Conclusions
Ocular ultrasonography is the most commonly used imaging technique for determining the presence of a uveal melanoma, especially in opaque ocular media when dilated fundus examination of the eye cannot be made. Ocular ultrasonography has many advantages in clinical practice due to availability, no exposure to radiation, and to low price.
In summary, this article presents the difficulties associated with the diagnosis of necrotic uveal melanoma in the absence of fundus eye examination due to ocular opaque media. In this case, we should do the differential diagnosis with other pathologies that could resemble echographically with uveal melanoma.
These pathologies may include:
-
Cavernous hemangioma – in case of extensive bleeding, hemangioma could mimic ultrasonographically an uveal melanoma.
-
Choroidal detachment associated with dense vitreous hemorrhage.
-
Vitreous hemorrhage, especially when it is massive.
-
Intraocular foreign body, when it is associated with inflammation or extensive bleeding.
-
Endoftalmitis, where we have multiple inflammation echoes.
-
Lymphoid tumor – if it is associated with vitreous accumulation of lymphocytes.
-
Lens luxated in vitreous posttraumatic associated with inflammation or bleeding.
-
Sarcoid nodules or tubercular granuloma associated with dense vitreous inflammation.
In cases when ultrasonography could not establish a clear diagnosis, we should use ancillary test.
Scott et al.(34) also compared the effectiveness of ultrasonography, magnetic resonance imaging and computed tomography, and revealed a 100% sensitivity for the ultrasound examination.
Martin et al.(35) suggested that ultrasonography should be an elementary tool in the initial diagnosis of choroidal melanoma and control during the treatment. Because of the high ultrasound penetration of light through ocular tissues, B-mode imaging is considered a more effective diagnostic process for tumors than anterior-segment optical coherence tomography (AS-OCT)(1). n
Abbreviations: UM = uveal melanoma; USG = ultrasonography; FFA = fundus fluorescein angiography; ICGA = indocyanine green angiography; OCT = optical coherence tomography; FAF = fundus autofluorescence; FNAB = fine-needle aspiration biopsy; OS = left eye; OD = right eye; MRI = magnetic resonance imaging; CT = computed tomography; AS-OCT = anterior-segment optical coherence tomography
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond). 2017;31(2):241-257.
-
Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38(5):549-553.
-
Souto EB, Zielinska A, Luis M, et al. Uveal melanoma: physiopathology and new in situ-specific therapies. Cancer Chemother Pharmacol. 2019;84(1):15-32.
-
Dogrusöz M, Jager MJ, Damato B. Uveal Melanoma Treatment and Prognostication [published correction appears in Asia Pac J Ophthalmol (Phila). 2017 May-Jun;6(3):305]. Asia Pac J Ophthalmol (Phila). 2017;6(2):186-196.
-
Blum ES, Yang J, Komatsubara KM, Carvajal RD. Clinical Management of Uveal and Conjunctival Melanoma. Oncology (Williston Park). 2016;30(1):29-48.
-
Tarlan B, Kıratlı H. Uveal Melanoma: Current Trends in Diagnosis and Management. Turk J Ophthalmol. 2016;46(3):123-137.
-
Waldron RG, Jang TB. B-Scan ocular Ultrasound. Medscape. https://emedicine.medscape.com/article/1228865-overview?form=fpf.
-
Mustafa M, Montgomery J, Atta H. A novel educational tool for teaching ocular ultrasound. Clin Ophthalmol. 2011;5:857-860.
-
Zhang Q, Yang H, Kang SJ, et al. In vivo high-frequency, contrast-enhanced ultrasonography of uveal melanoma in mice: imaging features and histopathologic correlations. Invest Ophthalmol Vis Sci. 2011;52(5):2662-2668.
-
Li W, Gragoudas ES, Egan KM. Tumor basal area and metastatic death after proton beam irradiation for choroidal melanoma. Arch Ophthalmol. 2003;12(1):68-72.
-
Richtig E, Langmann G, Mullner K, et al. Calculated tumour volume as a prognostic parameter for survival in choroidal melanomas. Eye. 2004;18(6):19-623.
-
Gass JD. Comparison of uveal melanoma growth rates with mitotic index and mortality. Arch Ophthalmol. 1985;103(7):924-931
-
Coleman DJ, Silverman RH, Rondeau MJ, Lizzi FL, McLean IW, Jakobiec FA. Correlations of acoustic tissue typing of malignant melanoma and histopathologic features as a predictor of death. Am J Ophthalmol. 1990;110(4):380-388.
-
Romero JM, Finger PT, Rosen RB, Iezzi R. Three-dimensional ultrasound for the measurement of choroidal melanomas. Arch Ophthalmol. 2001;119(9):1275-1282.
-
Cusumano A, Coleman DJ, Silverman RH, et al. Three-dimensional ultrasound imaging: clinical applications. Ophthalmology. 1998;105(2):300-306.
-
Solnik M, Paduszyńska N, Czarnecka AM, et al. Imaging of Uveal Melanoma-Current Standard and Methods in Development. Cancers (Basel). 2022;14(13):3147.
-
Oberg J, Spenger C, Wang FH, et al. Age related changes in brain metabolites observed by 1H MRS in APP/PS1 mice. Neurobiol Aging. 2008;29(9):1423-1433.
-
Garcia-Valenzuela E, Dahl AA, Choroidal Melanoma. Medscape. https://emedicine.medscape.com/article/1190564-overview?form=fpf.
-
Margo CE. The Collaborative Ocular Melanoma Study: an overview. Cancer Control. 2004;11(5):304-309.
-
Collaborative Ocular Melanoma Study Group. Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study: COMS report no. 21. Arch Ophthalmol. 2003;121(8):1163-1171.
-
Ossoinig KC. Clinical echo-ophthalmology. In: Grzybowski A (Ed.). Current Concepts of Ophthalmology. CV Mosby Co., St Louis, MO, USA, 1972; Volume III, pp. 101–130.
-
Damato B, Babic K. Experience with Ultrasonography in the Diagnosis and Treatment of Uveal Melanoma. Retina Today. https://retinatoday.com/articles/2013-nov-dec/experience-with-ultrasonography-in-the-diagnosis-and-treatment-of-uveal-melanoma.
-
Thareja S, Rashid A, Grossniklaus HE. Spontaneous Necrosis of Choroidal Melanoma. Ocul Oncol Pathol. 2014;1(1):63–9.
-
Bujara K. Necrotic malignant melanomas of the choroid and ciliary body. A clinicopathological and statistical study. Graefes Arch Clin Exp Ophthalmol. 1982;219(1):40–3.
-
Palamar M, Thangappan A, Shields CL, Ehya H, Shields JA. Necrotic choroidal melanoma with scleritis and choroidal effusion. Cornea. 2009;28(3):354–6.
-
Baumann C, Iannetta D, Coupland SE, Groenewald C, Vishwanath M, Heimann H. Spontaneous Necrosis of a Large Choroidal Melanoma: Unusual Presentation in a 49-Year-Old Male. Ocul Oncol Pathol. 2020;6(3):174-179.
-
Brannan S, Browne B, Clark BJ. Massive infarction of ocular tissues complicating a necrotic uveal melanoma. Eye (Lond). 1998;12(Pt 2):324-325.
-
Groenewald C, Konstantinidis L, Damato B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye (Lond). 2013;27(2):163-71.
-
Reese AB, Archila EA, Jones IS, Cooper WC. Necrosis of malignant melanoma of the choroid. Am J Ophthalmol. 1970;69(1):91-104.
-
Peddada K, Dalvin LA, Mashayekhi A, Shields CL. Diagnostic challenges in necrotic uveal melanoma. Retin Cases Brief Rep. 2022;16(1):92-94.
-
Sellam A, Desjardins L, Barnhill R, et al. Fine needle aspiration biopsy in uveal melanoma: technique, complications, and outcomes. Am J Ophthalmol. 2016;162:28–34.e1.
-
Uduma UF, Titalom K. Intra-orbital malignant melanoma: role of MR imaging (a case report and literature review). Glob J Health Sci. 2012;4(1):253-258.
-
Egeland TA, Gaustad JV, Galappathi K, Rofstad EK. Magnetic resonance imaging of tumor necrosis. Acta Oncol. 2011;50(3):427-434.
-
Scott IU, Murray TG, Hughes JR. Evaluation of imaging techniques for detection of extraocular extension of choroidal melanoma. Arch Ophthalmol. 1998;116(7):897-899.
-
Martin JA, Robertson DM. Extrascleral extension of choroidal melanoma diagnosed by ultrasound. Ophthalmology. 1983;90(12):1554-1559.