Introduction. Cancers of the head and neck, which include neoplasia of the ENT or upper respiratory and digestive tracts, represent a wide variety of malignant diseases originating in the cells of the upper aerodigestive tract. Oropharyngeal carcinoma represents 10-15% of head and neck neoplasms. Objective of the study. The purpose of this paper is to review recent data on several topics, including HPV-positive risk factors for oral squamous cell carcinoma (OSCC), guidelines in diagnostic evaluation, treatment, prognosis and prevention strategies. The purpose of the paper. A comparison of proliferation factors, degradation of the basal lamina and modulation of the connective tissue with the survival time of patients diagnosed with oropharyngeal neoplasm. Materials and method. The study analyzed a group of 272 patients hospitalized in the ENT Clinic of the Craiova County Emergency Clinical Hospital over a period of five years, between 1 January 2017 and 31 December 2021, diagnosed with oropharyngeal neoplasm. Results. The present study included a number of 272 malignant oropharyngeal tumors, of which the majority were represented by squamous cell oropharyngeal carcinomas; well differentiated (19 cases; 6.99%), moderately differentiated (130 cases; 47.79%), poorly differentiated (110 cases; 40.44%); LMNH (four cases; 1.47%), one leiomyosarcoma (0.36%), one malignant synovioma (0.36%) and one adenoid cystic carcinoma (0.36%). Two patients refused biopsy (0.73%) and four patients preferred that the histopathological examination be performed in the private system as well (1.47%). Conclusions. Oropharyngeal squamous cell carcinoma accounts for 95% of all head and neck cancers, and in the last decade its incidence has increased by 50%.
Histopathological and immunohistochemical aspects of the oropharyngeal neoplasm in the ENT Clinic, Craiova
Aspecte histopatologice şi imunohistochimice ale neoplasmului orofaringian în Clinica ORL din Craiova
First published: 30 mai 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ORL.59.2.2023.8111
Abstract
Rezumat
Introducere. Cancerele capului şi gâtului ce includ neoplaziile ORL sau ale căilor aeriene şi digestive superioare reprezintă o mare varietate de boli maligne cu originea în celulele tractului aerodigestiv superior. Carcinomul orofaringian reprezintă 10-15% din neoplaziile capului şi gâtului. Obiectivul lucrării. Scopul acestei lucrări este de a revizui datele recente despre mai multe subiecte, inclusiv factorii de risc HPV-pozitivi ai carcinomului orofaringian cu celule scuamoase (OSCC), alături de liniile directoare în evaluarea diagnosticului, tratamentului, prognosticului şi a strategiilor de prevenire. Scopul lucrării. Compararea factorilor de proliferare, alături de degradarea laminei bazale şi modularea ţesutului conjunctiv cu timpul de supravieţuire al pacienţilor diagnosticaţi cu neoplasm orofaringian. Materiale şi metodă. Studiul a analizat un grup de 272 de pacienţi internaţi în Clinica ORL a Spitalului Clinic Judeţean de Urgenţă din Craiova, pe o perioadă de cinci ani, între 1 ianuarie 2017 şi 31 decembrie 2021, diagnosticaţi cu neoplasm orofaringian. Rezultate. Acest studiu a cuprins un număr de 272 de tumori maligne orofaringiene, dintre acestea majoritatea fiind reprezentate de carcinoame orofaringiene cu celule scuamoase, bine diferenţiate (19 cazuri; 6,99%), moderat diferenţiate (130 de cazuri; 47,79%), slab diferenţiate (110 cazuri; 40,44%), LMNH (patru cazuri; 1,47%), un leiomiosarcom (0,36%), un sinoviom malign (0,36%) şi un carcinom adenoid chistic (0,36%). Doi pacienţi au refuzat biopsia (0,73%) şi patru pacienţi au preferat ca examenul histopatologic să fie efectuat şi în sistemul privat (1,47%). Concluzii. Carcinomul orofaringian cu celule scuamoase reprezintă 95% din toate formele de cancer de cap şi gât, iar în ultimul deceniu incidenţa acestuia a crescut cu 50%.
Introduction
Head and neck cancers, which include ENT or upper airway and digestive tract neoplasia, represent a wide variety of malignant diseases originating in the cells of the upper aerodigestive tract. Oropharyngeal carcinoma represents 10-15% of head and neck neoplasms(1).
In general, cancers, including oral squamous cell carcinoma (OSCC), arise from the accumulation of genetic alterations and epigenetic abnormalities in signaling pathways that are associated with cancer, resulting in phenotypes that facilitate the development of OSCC. Oral squamous cell carcinoma is a malignant neoplasm derived from the stratified squamous epithelium of the oral mucosa. Its pathogenesis is multifactorial, associated with cigarette smoke, alcohol and snuff, as well as the human papillomavirus (HPV)(2).
Cancer of the oral cavity is the sixth leading cause of cancer worldwide. In the United States of America alone, there are over 21,500 diagnoses of oral carcinomas each year and 6000 Americans die from it annually(3). The incidence of oral carcinoma varies worldwide, with estimates exceeding 40 per 100,000 in parts of France, southern Asia and Hungary. Ninety percent of these malignancies are squamous cell carcinomas.
Oral squamous cell carcinoma is by far the most important and frequent malignant neoplasm of the mucosa affecting the head and neck, and represents over 90% of all malignant neoplasms that develop in this region. 80-90% of head and neck cancers are thought to be associated with known risk factors such as smoking and alcohol abuse(4). However, 15-20% of head and neck cancers occur in people with no history of tobacco or alcohol use(5). We therefore believe that there are various etiological factors that interact in a multifactorial process for the induction and proliferation of neoplastic cells.
Oral malignant lesions develop through a series of histopathological stages: from average dysplasia (low grade), moderate and severe (high grade) to carcinoma in situ and then to invasive disease. The early detection of these premalignant oral lesions that will develop into invasive tumors is necessary to improve the prognosis of oral cancer. Due to the fact that there is no tool for delimiting the risk of development in the case of low-grade oral lesions, we cannot determine which of these cases require an aggressive surgical or medicinal intervention. Within these morphological processes, angiogenesis and lymphangiogenesis are essential processes for tumor growth and metastasis, because without a blood supply, incipient neoplasms cannot grow larger than 2 mm(3).
Materials and method
The present study included a number of 272 malignant oropharyngeal tumors, of which the majority were represented by squamous cell oropharyngeal carcinomas; well differentiated (19 cases; 6.99%), moderately differentiated (130 cases; 47.79%), poorly differentiated (110 cases; 40.44%); LMNH (four cases; 1.47%), one leiomyosarcoma (0.36%), one malignant synovioma (0.36%) and one adenoid cystic carcinoma (0.36%). Two patients refused biopsy (0.73%) and four patients preferred that the histopathological examination be performed in the private system as well (1.47%).
After clinical diagnosis and preoperative preparation, the patients received surgical treatment. The biological material collected from the surgical intervention rooms, respectively the tumor fragments, were sent to the pathological anatomy laboratory for histopathological diagnosis.
Immediately after harvesting, the biological material, right from the operating room, went into a 10% neutral formalin fixative solution for 48 hours at laboratory temperature in glass or plastic containers resistant to the action of formalin.
In our study, to evaluate the proliferative activity of cells from oral tumors, we used three immunohistochemical markers: PCNA, Ki67 and p53. For the evaluation of the angiogenesis process by immunohistochemistry techniques, there are several types of specific antibodies for marking endothelial cells: CD31, CD34, von Willebrand Factor (vW), CD105 (Podoplanin). In our study, we used an immunohistochemical marker specific for young endothelial cells – namely, the anti-CD34 antibody. This allowed us to highlight mature and immature blood capillaries. In fact, CD34-positive cells are considered endothelial precursor cells capable of forming new vessels in areas of tissue ischemia, initially unrelated to the preexisting vascular network.
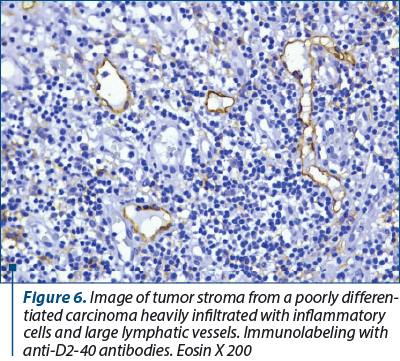
Regarding the process of lymphangiogenesis, it has a major contribution to the formation of new lymphatic vessels, through the proliferation and germination of endothelial cells from preexisting lymphatic vessels(6). Lymphangiogenesis is a dynamic process during embryogenesis, but is largely absent under normal, postnatal physiological conditions. In adult individuals, lymphangiogenesis occurs only under certain pathological conditions, such as inflammation, tissue repair and tumor growth(7-9).
In our study, we used a specific D2-40 antibody to identify the lymph nodes in the tumor stroma. D2-40 is a monoclonal antibody, a gm. 40,000 dalton sialoglycoprotein, that reacts with a fixation-resistant epitope from the lymphatic endothelium. This property of the epitope makes this immunomarker extremely useful for the study of lymphatic vessels on formalin-fixed and paraffin-embedded histological preparations.
Results
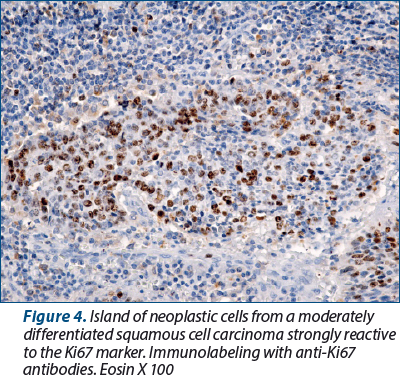
In the evaluation of cell proliferation factors, we started from the fact that human and animal tissues are made up of labile cells that have a lifespan limited to days, months or years, after which they enter a natural process of cell death, called apoptosis, process by which the tissue and the organism as a whole maintain a relatively constant number of cells for a certain stage of its development and existence, maintaining its tissue and organic architecture, which leads to the preservation of local and general homeostasis. In normal tissues, cell division and death are controlled by specific, highly complex, some incompletely known, genetically encoded mechanisms.
Unlike normal tissues, malignant tumors and some premalignant lesions are characterized by a significant change in the processes of cell division and maturation, by a profound disruption of the genetic program, which leads to the appearance of serious pathological processes. They disrupt the local homeostasis, then they alter the entire tissue, organ and organism, resulting in the appearance of the disease state. If normal cells have a well-defined mitotic rhythm and lifespan, cancer cells divide and grow uncontrollably, and the process of apoptosis is mostly altered.
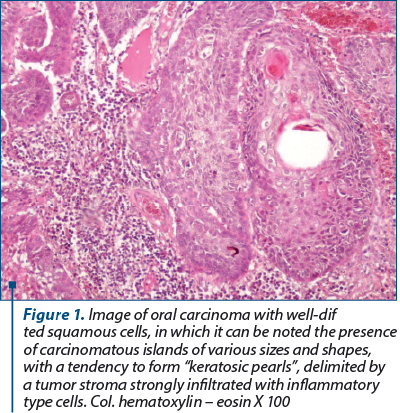
Well-differentiated squamous cell carcinomas were formed by well-differentiated squamous cells, arranged in islands of various shapes and sizes, with pearls of keratin inside them, resulting from a process of “neoplastic maturation”. Inside the keratotic pearls, the cells appeared acidophilic, with pyknotic nucleus, with karyolysis, while in the rest of the cells, the nucleus were of various shapes and sizes, much larger than the nucleus of the normal epithelium, with obvious nucleolus and unevenly arranged chromatin, sometimes in the form of grunts. The shape and sizes of the neoplastic islands, as well as the keratotic pearls were highly variable.
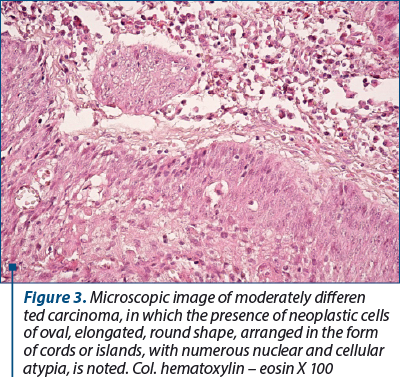
Moderately differentiated carcinomas were formed by cords or islands of atypical, neoplastic, oval, elongated, round epithelial cells infiltrating the fibrous tumor stroma. At their periphery, the carcinomatous islands were delimited by fibrous stromal elements or inflammatory type cells. The nucleolus of the neoplastic cells had various shapes and sizes, most of them being hypochromic with large nucleolus.
Tumor cells frequently appeared as atypical cells dispersed diffusely in the stroma of the oral, lingual or labial mucosa, with rare intercellular bridges. Often, the tumor cells showed large, deformed, hyperchromic or hypochromic nucleolus, with incisions and buds, with multiple atypical mitoses. The cytoplasm of these cells appeared acidophilic, inhomogeneous, and the cell membrane showed inhomogeneous digitiform extensions, characteristic of cellular pleiomorphism.
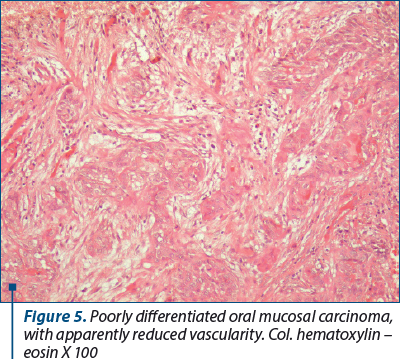
Poorly differentiated cell carcinomas appeared as cell cords, islands, or epithelioid-appearing cells of various shapes and sizes, bearing no resemblance to the epithelium from which they arose. These different aspects of cancer cells lead us to believe that malignant tumors are heterogeneous, multicellular entities containing multiple cell lineages whose interactions with each other and with the extracellular matrix through paracrine secreted soluble molecules are dynamic and favor cell proliferation, movement and differentiation malignancies.
The tumor stroma resulting from the transformation of the chorion of the covering epithelium into stroma with particular characters, as an adaptation reaction of the innate mesenchyme to the tumor invasion, was most often infiltrated with inflammatory type cells. In this stroma, numerous large-caliber, congested blood vessels were highlighted, mainly arranged around the islands of neoplastic cells. Due to the high vascular density, this stroma has the role of nutrition and support for tumor cells. It is well known that, immediately after the rupture of the basement membrane and the penetration of cancer cells into the underlying connective tissue, an inflammatory reaction is triggered which will lead to the formation of a particular granulation tissue that will mature and complete a tumor connective tissue, also known as tumoral stroma.
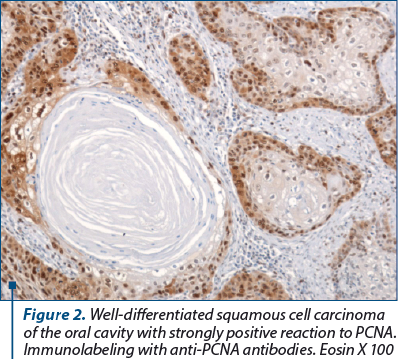
In our study, all squamous cell carcinomas, regardless of the degree of differentiation, were intensely PCNA-positive, except for areas of “keratotic pearls” in well-differentiated squamous cell carcinomas. More than 90% of the nucleolus of the neoplastic cells were intensely positive for anti-PCNA antibodies. In other words, almost all tumor cells have the ability to multiply. In areas with keratotic pearls, the number of positive cells gradually decreased as they involuted. That is why we consider that the reaction to PCNA is extremely valuable when the difference between a simple dysplasia and a carcinoma in situ has to be made, the reaction being intensely positive when neoplastic proliferation is triggered.
In our study, we tried to evaluate not only the presence of angiogenesis vessels in oral squamous carcinoma, but also their number and the area occupied by these vessels in the different forms of oral carcinomas. Where the tumor stroma was heavily infiltrated with inflammatory type cells, more angiogenesis vessels of a much larger caliber were identified than in the fibrous tumor stroma. In the stroma dominated by collagen fibers, the number and size of angiogenesis vessels were much lower than in areas of tumor proliferation where the increased number of angiogenesis vessels correlated with the intensity of the chronic inflammatory infiltrate. These aspects we observed confirm the idea that angiogenesis is stimulated by cells of the immune system that are able to synthesize and secrete numerous factors with angiogenic potential.
The number of angiogenesis vessels in poorly differentiated carcinomas was approximately 166 vessels/mm2, in moderately differentiated carcinoma – approximately 88 vessels/mm2, and in well-differentiated carcinoma – 72 vessels/mm2. The vascular areas were also variable according to the degree of differentiation of the carcinoma. Thus, in poorly differentiated carcinoma it was about two times higher than in moderately differentiated carcinoma and about four times higher than in well-differentiated carcinoma.
Discussion
Chronic exposure to carcinogens causes genetic abnormalities in the cells of the oral mucosa. When these genetic anomalies determine the activation of proto-oncogenes and the inactivation of tumor suppressor genes, cells acquire new properties of growth and multiplication. It is clearly accepted by everyone that oral squamous cell carcinoma develops as a result of the accumulation of genetic errors in the same tissue.
Changes affecting the TP53 gene cause the accumulation of an abnormal protein in the nucleus, which can be highlighted by immunohistochemistry. Some studies have indicated that, in the oral cavity, the presence of p53 positive cells in the upper layers of the dysplastic epithelium may imply a risk of evolution towards carcinoma(10).
Evidence that the p53 pathway is very important in the biology of oral cancer has led to the use of such immunohistochemical analysis as a simple, rapid and inexpensive method in the case of potentially malignant lesions in an attempt to find a useful marker for predicting progression to oral squamous cell carcinoma.
The presence of large-caliber lymphatic vessels denotes their ability to transport tumor cells to the regional lymph nodes. In recent years, a large number of authors have reported the existence of a correlation between VEGF-C expression, lymphangiogenesis and metastasis in regional lymph nodes(11). According to some authors, there is a strong correlation between the density of lymphatic vessels and the expression of VEGF-C. The same study shows that there is no direct correlation between the density of lymphatic vessels and the presence of lymph node metastases(12).
Cancer epidemiology claims that 20-40% of tumors are caused by smoking, 2-4% are caused by excessive alcohol consumption, 10-70% are caused by the diet and lifestyle of the patients, 0.5-3% are determined by medicinal substances, 1-10% are determined by viral infections and 1-5% are determined by atmospheric pollution, therefore a significant percentage of cancers are mainly determined by the patients’ lifestyle, cancer becoming, from this point of view, a condition that also benefits from “prophylactic treatment”(13).
Conclusions
1. The present study included a number of 272 oropharyngeal malignant tumors, of which the majority were represented by squamous cell oropharyngeal carcinomas; well differentiated (19 cases; 6.99%), moderately differentiated (130 cases; 47.79%), poorly differentiated (110 cases; 40.44%); LMNH (four cases; 1.47%), one leiomyosarcoma (0.36%), one malignant synovioma (0.36%) and one adenoid cystic carcinoma (0.36%). Two patients refused biopsy (0.73%) and four patients preferred that the histopathological examination be performed in the private system as well (1.47%).
2. Well-differentiated squamous cell carcinomas were formed by well-differentiated squamous cells, arranged in islands of various shapes and sizes, with keratin pearls inside them, resulting from a process of “neoplastic maturation”.
3. Moderately differentiated carcinomas were formed by cords or islands of atypical, neoplastic, oval, elongated, round epithelial cells infiltrating the fibrous tumor stroma. At their periphery, the carcinomatous islands were delimited by fibrous stromal elements or inflammatory type cells. The nucleus of the neoplastic cells had various shapes and sizes, most of them being hypochromic with large nucleolus.
4. Poorly differentiated cell carcinomas appeared as cell cords, islands, or epithelioid-appearing cells of various shapes and sizes, bearing no resemblance to the epithelium from which they arose.
5. The number of angiogenesis vessels in poorly differentiated carcinomas was approximately 166 vessels/mm2, in moderately differentiated carcinoma – approximately 88 vessels/mm2, and in well-differentiated carcinoma – 72 vessels/mm2. The vascular areas were also variable according to the degree of differentiation of the carcinoma. Thus, in poorly differentiated carcinoma it was about two times higher than in moderately differentiated carcinoma and about four times higher than in well-differentiated carcinoma.
6. All squamous carcinomas, regardless of the degree of differentiation, were intensely positive for PCNA, except for areas of “keratotic pearls” in well-differentiated squamous carcinomas. More than 90% of the nucleolus of the neoplastic cells were intensely positive for anti-PCNA antibodies. In other words, almost all tumor cells have the ability to multiply.
7. Numerous studies have shown that in solid tumors an abnormal vasculature appears, which is a histopathological hallmark, where blood vessels are arranged adjacent to the tumor itself(14). The higher number of tumor vessels increase the risk of tumor cells entering the systemic circulation and giving distant metastases.
8. In addition to increasing the density of lymphatic vessels, lymphangiogenic growth factors also act to enlarge and dilate lymphatic vessels(15). Interestingly, the activation of VEGFR-2 causes expansion of lymphatic vessels, in contrast to VEGFR-3 which activates lymphangiogenesis and vascular sprouting more(16). The enlarged lymphatic vessels that drain the tumor allow the migration and metastasis of tumor cells towards the lymph node, most likely through the increase of lymphatic flow(17), and facilitate the metastasis of some groups of tumor cells.
9. The anatomical and clinical types of oropharyngeal cancer are dictated by the anatomical and histological characteristics of the region(18).
Acknowledgment: This work was supported by the grant POCU/993/6/13/153178, “Performanţă în cercetare” (“Research performance”), co-financed by the European Social Fund within the Sectorial Operational Program Human Capital 2014-2020.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
-
Moncrieff M, Sandilla J, Clark J, Clifford A, Shannon K, Gao K, O’Brien C. Outcome of primary surgical treatment of T1 and T2 carcinomas of the oropharynx. Laryngoscope. 2009;119(2):307–311.
-
Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol Lett. 2014;8(1):7-11.
-
Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998 [published correction appears in CA Cancer J Clin 1998 May-Jun;48(3):192] [published correction appears in CA Cancer J Clin 1998 Nov-Dec;48(6):329]. CA Cancer J Clin. 1998;48(1):6-29. doi:10.3322/canjclin.48.1.6.
-
Licitra L, Bernier J, Grandi C, Merlano M, Bruzzi P, Lefebvre JL. Cancer of the oropharynx. Crit Rev Oncol Hematol. 2002;41(1):107-122. doi:10.1016/s1040-8428(01)00129-9.
-
Gillison ML, Shah KV. Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol. 2001;13(3):183-8.
-
He Y, Rajantie I, Ilmonen M, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64(11):3737-3740.
-
Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126(10):2167-2177.
-
Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6(3-4):109-122.
-
Srinivasan RS, Geng X, Yang Y, et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24(7):696-707.
-
Montebugnoli L, Felicetti L, Gissi DB, et al. Predictive Role of p53 Protein as a Single Marker or Associated to Ki67 Antigen in Oral Carcinogenesis. Open Dent J. 2008;2:24-29.
-
Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21(7):1104-1117.
-
Ohta Y, Shridhar V, Bright RK, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999;81(1):54-61.
-
Vrînceanu D. Probleme de diagnostic şi tratament în cancerul amigdalei palatine. PhD Thesis, Bucharest, 2007.
-
Matsuda Y, Hagio M, Ishiwata T. Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol. 2013;19(1):42-48.
-
Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8(9):747-757.
-
Wirzenius M, Tammela T, Uutela M, et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med. 2007;204(6):1431-1440.
-
Hoshida T, Isaka N, Hagendoorn J, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66(16):8065-8075.
-
Obreja S, Ioniţă E, Mitroi M, Ioniţă I. Lexicon al diagnosticului în otorinolaringologie – cancerul faringelui bucal (orofaringian), pg. 245-249; cancerul limbii, pg. 260-262. Ed. Didactică şi Pedagogică R.A., Bucureşti, 1998.