Introduction. Cognitive decline is a frequent consequence of stroke. This study aims to assess the contribution of admission point-of-care (POC) biomarkers to three-month cognitive decline and quality of life (QoL) in spontaneous intracerebral hemorrhage (sICH) patients.
Materials and method. This prospective observational study included adult patients diagnosed with sICH within the first eight hours from symptom onset, over a period of 18 months. Medical history and clinical and paraclinical data were collected during the emergency department presentation (imagistics and POC biomarkers – complete blood count, glucose, renal and hepatic function). The follow-up included clinical assessment on the second and seventh days, a control computed tomography and telephone interviews on the 90th day.
Results. All 35 enrolled patients reported alterations of quality of life. The quality of life was influenced by lymphocytes, platelets and D-dimer, with lymphocytes and D-dimer associated with mobility, self-care and ability to perform usual tasks. QoL related to pain/discomfort was influenced by D-dimer only. The median TICS-m was 23, with 13 (72.22%) evaluated participants reaching moderate cognitive impairment scores. Cognitive performance in general was influenced by mean platelets volume. Category fluency was not influenced by any parameter.
Discussion. sICH leads to significant morbidity, with nearly 74% of all participants either dead or cognitively impaired on follow-up. Bearing in mind the simplicity of routinely obtaining POC inflammatory markers, future adequately sized studies should further explore their utility on predicting QoL and cognitive decline.
Conclusions. Emergency department POC biomarkers could contribute to a timely estimation of cognitive impairment and QoL in sICH patients.
Screening precoce al declinului cognitiv şi al calităţii vieţii după hemoragia spontană intracerebrală
Emergency department point‑of-care predictors of cognitive decline and quality of life after spontaneous intracerebral hemorrhage
First published: 18 aprilie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.68.1.2022.6307
Abstract
Rezumat
Introducere. Declinul cognitiv este o consecinţă frecventă a accidentului vascular cerebral (AVC). Acest studiu a avut ca scop evaluarea relaţiei dintre o serie de biomarkeri evaluaţi la locul de îngrijire (POC) şi declinul cognitiv şi al calităţii vieţii (QoL) la trei luni de la AVC hemoragic spontan.
Materiale şi metodă. Studiul (prospectiv, observaţional) a inclus, pentru o perioadă de 18 luni, pacienţi adulţi cu AVC hemoragic evaluaţi în primele opt ore de la debutul simptomatologiei. Istoricul şi datele clinice şi paraclinice au fost înregistrate la prezentarea în departamentul de urgenţă (imagistică, biomarkeri POC – hemoleucogramă, glicemie, date paraclinice privind funcţia hepatică şi renală). Evaluarea în dinamică a inclus evaluare clinică în zilele 2 şi 7, tomografie computerizată de control şi interviuri clinice prin telefon în ziua 90.
Rezultate. Toţi cei 35 de pacienţi incluşi au raportat o deteriorare a calităţii vieţii. QoL s-a corelat cu numărul de limfocite, trombocite şi D-dimeri, numărul de limfocite şi D-dimerii fiind corelate cu subscalele de mobilitate, autoîngrijire şi cu abilitatea de a realiza sarcini uzuale. Subscala de durere/disconfort a fost corelată doar cu D-dimerii. Scorul median TICS a fost de 23, cu 13 (72,22%) dintre pacienţi prezentând scoruri de declin moderat. Performanţa cognitivă a fost corelată cu volumul plachetar mediu. Fluenţa verbală nu a fost corelată cu niciun parametru.
Discuţie. Accidentul vascular cerebral hemoragic conduce la o morbiditate semnificativă, aproximativ 74% dintre pacienţi decedând sau prezentând declin cognitiv la evaluarea în dinamică. Având în vedere simplitatea obţinerii de date POC, studii viitoare mai mari ar trebui să exploreze utilitatea acestor biomarkeri în predicţia declinului cognitiv şi al QoL.
Concluzii. Biomarkerii POC obţinuţi în departamentul de urgenţă pot contribui la estimarea precoce a declinului cognitiv şi al QoL la pacienţii cu AVC hemoragic.
Introduction
Cognitive decline is a frequent consequence of stroke, ranging between 37% and 71%(1,2). Up to 75% suffer from early post‑stroke cognitive impairment(2), with every third patient presenting post-stroke dementia by day 90(3) and cognitive disability during the first four years(4). One in five survivors has been diagnosed with dementia at six months after intracerebral hemorrhage (ICH), the annual rate later dropping at 5-8%(5). In ICH patients, there is little understanding of cognitive progression after the onset of the condition, possibly due to the still rather high early mortality rate(6,7). Nevertheless, precognitive impairment was found in 18% of ICH patients, including 9% of previously demented patients(8).
The development of subsequent cognitive deterioration has been predicted by older age, female gender, lower educational status(1), preexisting stroke or cognitive impairment(9), the severity of ICH (expressed as Glasgow Coma Scale [GCS] and/or National Institutes of Health Stroke Scale [NIHSS])(1), ICH volume and location(1,10), disseminated cortical siderosis and cerebral microbleeds(1), dominant hemisphere lesion(1,2), high systolic blood pressure (SBP) and red blood cells (RBC) indices(2). Significant cortical atrophy and leukoaraiosis have been linked with previous existing dementia(4), whilst a positive history of ischemic stroke and the hemorrhage volume have been independently associated with previous cognitive impairment(8). Cerebral atrophy was linked to the occurrence of post‑stroke cognitive impairment(11), its severity predicting cognitive decline(1), even in patients without preexisting cognitive impairment(9). However, cerebral atrophy had a protective role in medium basal ganglia hemorrhage(12).
The subjective assessment of quality of life in spontaneous ICH (sICH) patients constitutes a recent research interest and it includes personal estimations on health status, social activity, sleep quality, professional activity and family interaction(4). At three months, only a minority of ICH patients (13%) consider their health as being perfect(13), with age, severity of symptoms (higher NIHSS scores and larger baseline hematoma), intraventricular hemorrhage (IVH) presence and non-lobar hematomas representing independent predictors of a poor quality of life following ICH(4).
There is a paucity of data regarding the particular contribution of neuroinflammation to cognitive performance after sICH. Neuroinflammation, expressed as higher white blood cells (WBC)(1) and C‑reactive protein (CRP)(1,14,15) levels, has been identified as prognostic factor for cognitive impairment after ICH. Admission hyperglycemia has been associated with dementia(3). Abnormal RBC indices (higher mean corpuscular volume in particular) were associated with the development of early post‑stroke cognitive impairment in ICH patients and with a smaller chance of cognitive recovery(2). Lower lipidic values have been associated with dementia following stroke(3), considering the involvement of lipids in neurologic repairing processes, immunity response, cellular metabolism and preventing the fragility of cerebrovascular endothelium(16).
Telephone Interview for Cognitive Status – modified (TICS-m) is an instrument employed for global cognition assessment, including orientation, memory, attention, calculation and language assessment. The scale has been previously validated and used in post‑stroke patients(17). Various TICS-m versions have been used to describe cognitive status(18), with total scores varying from 27 to 50 and estimations that score adjustments based on education status, age and gender might increase accuracy(18,19). Nevertheless, it is considered a screening tool for mild cognitive impairment, rather than a diagnostic one(19).
The aim of this study is to assess the contribution of admission point-of-care (POC) biomarkers to three-month cognitive decline and quality of life in spontaneous intracerebral hemorrhage patients.
Materials and method
The present research is a prospective observational study, including adult patients (>18 years old) who were diagnosed with sICH within the first eight hours from symptoms’ onset. The study was conducted over a period of 18 months (December 2016 – June 2018). The details of the recruitment and the follow-up process have been previously published(20,21).
During the emergency department presentation, medical history and clinical and paraclinical data were collected, including routine POC venous biomarkers (complete blood count [CBC] – WBC, granulocytes [GRA], lymphocytes [LYM], middle sized cells [MID], PLT [platelets; and indices), glucose, renal and hepatic function) and imagistics (hematoma volume and location, perihematomal oedema and its size, IVH, mass effect, midline shift). The eligible patients underwent a clinical assessment (NIHSS) and, subsequently, blood samples for study biomarkers were collected, both POC (high-sensitive CRP, cardiac troponin I [cTnI] and D-dimer) and conventional determinations (fibrinogen, total and low-density lipoprotein [LDL] cholesterol, triglycerides). Derived inflammatory indexes were calculated, as previously published(21).
The follow-up was performed on the second and seventh days and at discharge (as clinical assessment). A control computed tomography (CT) scan was performed, ideally on the second day. On the 90th day, telephone interviews were conducted on functional outcome (FO) – modified Rankin Scale (mRS), independence – Barthel Index (BI), quality of life – EuroQoL 5D 5L, mood – Zung Depression Scale, cognition – TICS-m and verbal fluency (animal naming), for assessing global cognition and executive function.
In our research, TICS-m was a 44-point scale, containing the following items: address (3 points), hospital locations (2 points), date and time (5 points), age (1 point), telephone number (1 point), immediate recall of a 10-word list (10 points), subtracting by seven (5 points), counting backwards (1 point), responsive naming (2 points), current president and prime minister of Romania (2 points), word opposition (1 point), repetition of phrase (1 point), delayed recall of the 10-word list (10 points). Verbal fluency was assessed by animal naming for the duration of 1 minute. Mild cognitive impairment was defined as a TICS-m score below 27 or category fluency score below 10; dementia was defined as a TICS-m score below 20(17).
Quality of life assessment included five aspects (mobility, self-care, usual tasks, pain/discomfort, and anxiety/ depression), along with a subjective health state appreciation (ranging from 0 to 100). Each aspect received a grading ranging from 0 (no problem) to 4 (unable). The final EuroQoL 5D 5L score was a summation of each component.
Telephone interviews were conducted by instructed research assistants, but they were not blinded to patient’s condition.
The statistical calculations were performed in R version 4.1.2 (2021-11-01) – “Bird Hippie”, using the RStudio version 2021.09.2 Build 382 package. The normality of data distribution was tested using the Kolmogorov-Smirnov test. Centrality parameters were calculated accordingly (either mean ± standard deviation or median and quartiles). The c2 or Fisher’s exact tests were used to test the distribution of quantitative variables across groups. The Pearson’s or Spearman’s rho with correlation tests were used to test correlations between variables. Finally, the Mann-Whitney U or Kruskall-Wallis tests were used to test the presence of differences in the distributions of quantitative data across groups.
Results
A cohort of 35 patients have been prospectively enrolled over the 18 months of the study. Early mortality (defined on the seventh day) was 20%. By the 90th day, 12 of the 35 participants were deceased (34%) and only three of the survivors were living unaccompanied in their own homes, as opposed to 19 (83%) living together with family members. One participant was not available for telephone interview and, for other four, the caretaker completed the survey, hence TICS-m and category fluency were not possible.
The main demographic and clinical characteristics of study sample are presented in Table 1.
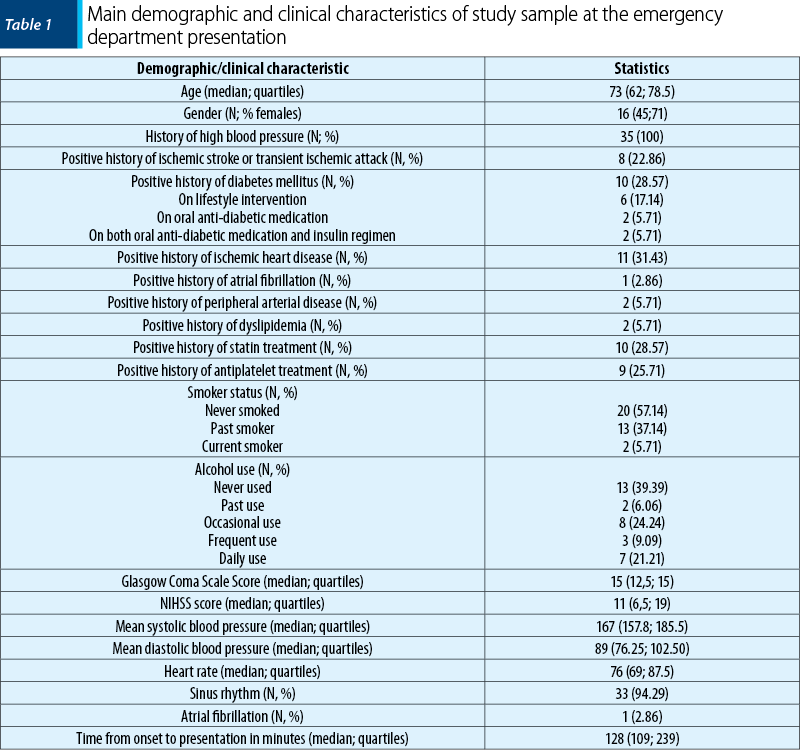
Half of the participants (18; 51%) were above 70 years old at the moment of the sICH diagnosis. The most frequent risk factors were hypertension, ischemic heart disease, diabetes mellitus and statin treatment. A pre-sICH dependency (defined as a mRS score of 1) was present in five participants. No patient had a positive history of hemorrhagic stroke or venous thromboembolism.
Cerebral computed tomography findings are summarized in Table 2.
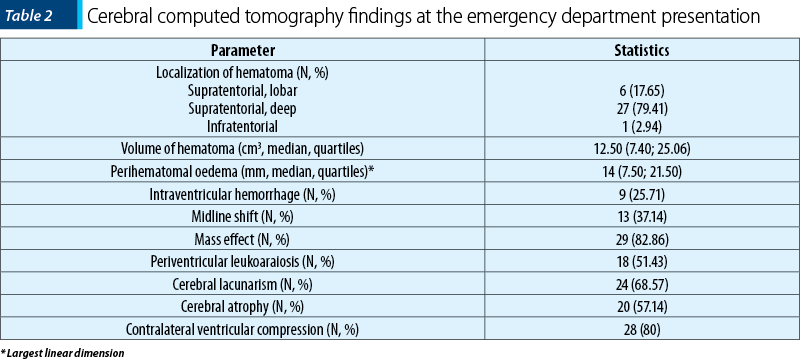
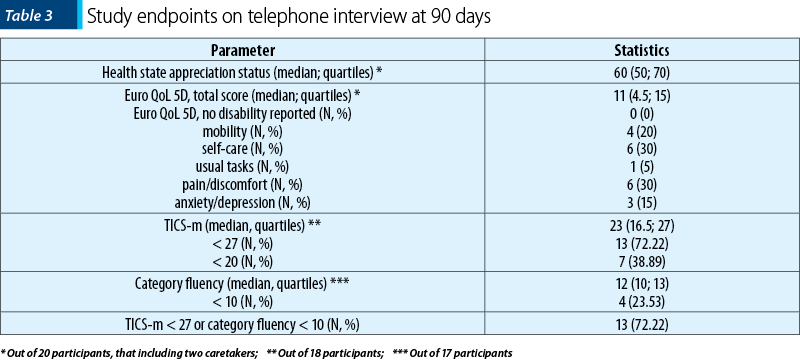
The vast majority of participants had a supratentorial deep hematoma, with a volume of less than 30 cm3 (28; 80%). The median values of study endpoints are presented in Table 3.
The correlation of admission demographic, clinical and paraclinical parameters with the study endpoints is presented in Table 4.
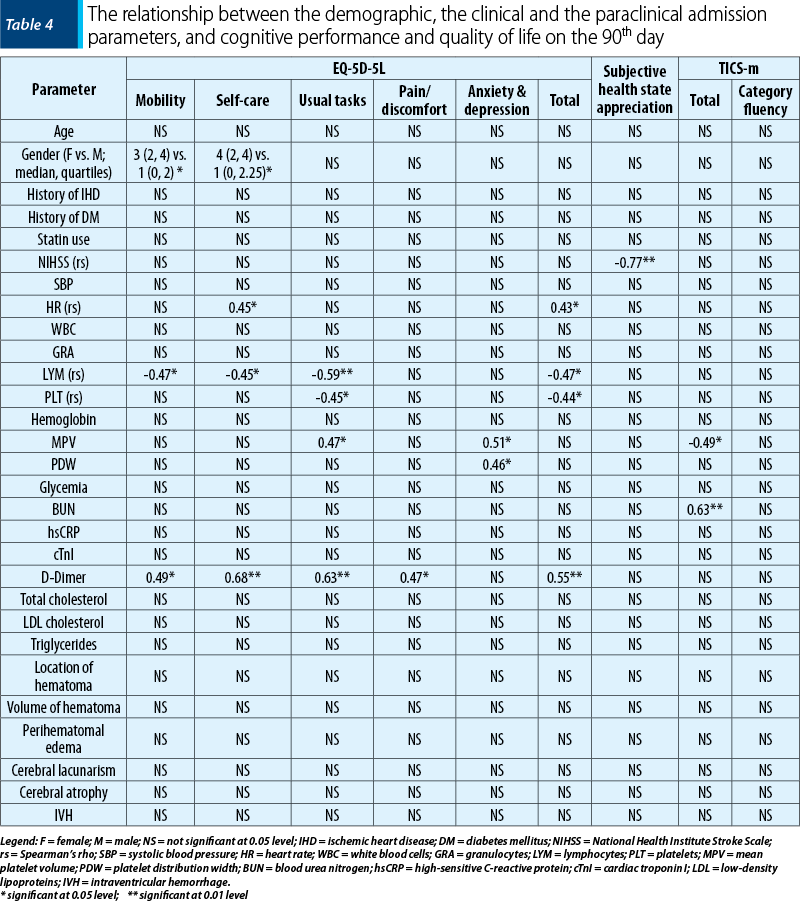
Quality of life per total score was influenced by heart rate, LYM, PLT and D-dimer values, with LYM and D-dimer associated with components such as mobility, self-care and ability to perform usual tasks. Quality of life related to pain or discomfort was influenced by D-dimer values only. The subjective evaluation of health status was influenced by NIHSS scores at admission.
Cognitive performance in general (TICS total score) was influenced by mean PLT volume (MPV) and blood urea nitrogen (BUN).
Category fluency, as expression of working memory, was not influenced by any of the parameters included in this study.
Discussion
Spontaneous intracerebral hemorrhage leads not only to high mortality rates, but also to significant morbidity, when considering the quality of life and cognition. At 90 days, nearly 74% of the evaluated patients were either dead or cognitively impaired, a proportion larger than the one from previous reports(1,2,17). Identifying early prediction markers (including blood biomarkers) could conclude in patients at risk, being provided access to more timely or better rehabilitation treatments. Emergency department point-of-care values of D-dimer, lymphocytes and platelets associated parameters have been significantly correlated with various aspects of quality of life and with cognition assessment. In contrast, the lipidic profile determined upon admission (total cholesterol, LDL cholesterol and triglycerides) failed to indicate any study endpoint.
All patients have reported an alteration of quality of life, although aspects such as self-care and pain/discomfort were reported as unaffected by every third patient. D-dimer has been reported as predicting poor three‑month functional outcome in sICH patients(22,23). A previous study we performed on the same cohort supports this finding(21) and observed that admission D-dimer also reflects the independence status on the 90th day (expressed as Barthel Index). When referring to cognitive decline, admission D-dimer has been reported as indicative of lower psychomotor speed and poorer verbal memory in COVID-19 patients(24), presumably as a consequence of cerebral hypoxia due to the vascular impairment generating increased D-dimer values. In our research, D-dimer significantly associated with poorer life quality (as a total EuroQoL 5D 5L score, but also all the individual items, except for anxiety/depression), yet failed to predict cognitive decline.
Although sICH-induced neuroinflammation has been associated with cognitive impairment(1,3,13,14), these reactive processes are believed not to have a significant contribution to cognitive deterioration, as it is in COVID-19(24). Our cohort unfortunately failed to support the impact of neuroinflammation on cognitive alterations following sICH, with none of the inflammatory parameters (including derived indexes and serum glucose) associating with neither cognition, nor with subjective health state assessment.
Lymphopenia was associated with adverse three‑month functional outcome in sICH(25,26), although previous studies on present cohort failed to determine significant differences between outcome groups(21). Currently, we have identified an association between admission lymphopenia and poorer quality of life after sICH, both as a total score and on separate items (mobility, self-care, performing usual tasks). Lymphopenia upon presentation is considered an expression of immunosuppression(25), with high neutrophil levels being involved in secondary neurologic injury(26), yet the present results do not enable us to estimate on the responsible pathophysiologic mechanism of LYM impacting post-sICH quality of life.
Bearing in mind the simplicity of routinely obtaining CBC or glucose measurements (including POC), future adequately sized studies should further explore their utility on cognitive decline prediction.
In our study, PLT and platelet volume indices, namely MPV and platelet distribution width (PDW), have been associated with quality-of-life score (and some of its individual components) and cognitive status, but with opposite associations, as presented in Table 4 (reduced number of PLT, larger mean volume and larger distribution width). We do not find this diversity of correlations as irrelevant, but rather as a valid argument that it is not only the number, but also the functionality of PLT that impacts homeostasis. Larger PLT are more hemostatically active(27), hence potentially impacting the progression of hemorrhage volume, which is eventually leading to a better quality of life following sICH. Furthermore, we would like to emphasize the vast array of information provided by standard ED POC CBC analyzers on PLT and RBC functionality, hence offering multiple opportunities for identifying potential sICH outcome predictors.
Significantly lower admission cholesterol(3), LDL(3) and high-density lipoproteine(28) cholesterol and triglycerides(3) levels have been associated with dementia following stroke(3). On the same cohort, the lipidic profile previously associated with three-month FO(21), yet the current analysis failed to determine any impact of reduced lipid levels as indicating cognitive impairment or affected quality of life.
Admission NIHSS is known to be significantly higher in patients with cognitive impairment(15), yet in our research it only inversely correlated with the subjective evaluation of health status. As the severity of sICH resides within the original size and location of the hematoma(4), we might speculate on it being the common denominator of admission sICH severity and global health status.
We consider certain associations of present study as rather random, such as those on gender, hearth rate and BUN. Female gender(1) and hearth rate(17) have been associated with cognitive decline following ICH, yet our findings contradict the existing evidence.
In spite of the novelty of current results and the practicality of ED POC testing, the current findings must be cautiously considered relevant, as the study sample was not large enough for formulating firm conclusions and the results were not adjusted to the education background of the patients and to preexisting dementia. Furthermore, the cognition assessment cut-off values discussed in this report have been arbitrarily selected from the existing literature (as opposed to internally determined), yet the proportion of cognitively impaired patients is nevertheless a high one. Lastly, the telephone interviewers were not blinded of patient progression and one patient was not available for telephone assessment on the 90th day.
Conclusions
Emergency department point-of-care biomarkers could contribute to a timely estimation of cognitive impairment and quality of life in spontaneous intracerebral hemorrhage patients. D-dimer, lymphocytes and platelet related parameters significantly correlated to EuroQoL 5D 5L score and with various of its components.
Disclaimer: The authors declare no conflicts of interest.
Bibliografie
-
Kazim SF, Ogulnick JV, Robinson MB, Eliyas JK, Spangler BQ, Hough TJ, et al. Cognitive Impairment After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. World Neurosurg. 2021;148:141–62.
-
Gong L, Gu Y, Yu Q, Wang H, Zhu X, Dong Q, et al. Prognostic Factors for Cognitive Recovery Beyond Early Poststroke Cognitive Impairment (PSCI): A Prospective Cohort Study of Spontaneous Intracerebral Hemorrhage. Front Neurol. 2020;11:278.
-
Barba R, Martínez-Espinosa S, Rodríguez-García E, Pondal M, Vivancos J, Del Ser T. Poststroke Dementia: Clinical Features and Risk Factors. Stroke. 2000;31(7):1494–501.
-
Pinho J, Costa AS, Araújo JM, Amorim JM, Ferreira C. Intracerebral hemorrhage outcome: A comprehensive update. J Neurol Sci. 2019;398:54–66.
-
Biffi A, Bailey D, Anderson CD, Ayres AM, Gurol EM, Greenberg SM, et al. Risk Factors Associated With Early vs Delayed Dementia After Intracerebral Hemorrhage. JAMA Neurol. 2016;73(8):969.
-
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009; 373(9675):1632–44.
-
Otite FO, Khandelwal P, Malik AM, et al. Ten-Year Temporal Trends in Medical Complications After Acute Intracerebral Hemorrhage in the United States. Stroke. 2018;48:596-603.
-
8. Laible M, Horstmann S, Möhlenbruch M, Schueler S, Rizos T, Veltkamp R. Preexisting cognitive impairment in intracerebral hemorrhage. Acta Neurol Scand. 2017;135(6):628–34.
-
Benedictus MR, Hochart A, Rossi C, Boulouis G, Hénon H, van der Flier WM, et al. Prognostic Factors for Cognitive Decline After Intracerebral Hemorrhage. Stroke. 2015;46(10):2773–8.
-
Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Hénon H, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 2016;15(8):820–9.
-
Casolla B, Caparros F, Cordonnier C, Bombois S, Hénon H, Bordet R, et al. Biological and imaging predictors of cognitive impairment after stroke: a systematic review. J Neurol. 2019;266(11):2593–604.
-
Kwon SM, Choi K-S, Yi H-J, Ko Y, Kim Y-S, Bak K-H, et al. Impact of brain atrophy on 90-day functional outcome after moderate-volume basal ganglia hemorrhage. Sci Rep. 2018;8(1):4819.
-
Christensen MC, Mayer S, Ferran J-M. Quality of Life After Intracerebral Hemorrhage: Results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) Trial. Stroke. 2009;40(5):1677–82.
-
Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80.
-
Nakase T, Sasaki M, Yoshioka S, Ikeda Y, Suzuki A. Risk of Cognitive Impairment in Acute Phase of Intracerebral Haemorrhage. Int J Stroke. 2013;8(4):E15.
-
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–16.
-
Ankolekar S, Renton C, Sare G, Ellender S, Sprigg N, Wardlaw JM, et al. Relationship between Poststroke Cognition, Baseline Factors, and Functional Outcome: Data from “Efficacy of Nitric Oxide in Stroke” Trial. J Stroke Cerebrovasc Dis. 2014;23(7):1821–9.
-
Lindgren N, Rinne JO, Palviainen T, Kaprio J, Vuoksimaa E. Prevalence and correlates of dementia and mild cognitive impairment classified with different versions of the modified Telephone Interview for Cognitive Status (TICS‐m). Int J Geriatr Psychiatry. 2019;34(12):1883–91.
-
Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJH, Petersen RC, et al. Validation of the Telephone Interview for Cognitive Status-modified in Subjects with Normal Cognition, Mild Cognitive Impairment, or Dementia. Neuroepidemiology. 2010;34(1):34–42.
-
Mureşan E-M, Golea A, Bolboacă SD, Perju-Dumbravă L. Feasibility of a pilot study on point-of-care biomarkers in spontaneous intracerebral hemorrhage in an emergency setting. Med Pharm Rep. 2021;64(3):307-17.
-
Mureşan E-M, Golea A, Vesa Ştefan, Lenghel M, Csutak C, Perju‑Dumbravă L. Emergency department point‑of‑care biomarkers and day 90 functional outcome in spontaneous intracerebral hemorrhage: A single‑center pilot study. Exp Ther Med. 2022;23(3):200.
-
Delgado P, Alvarez-Sabin J, Abilleira S, Santamarina E, Purroy F, Arenillas JF, et al. Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2006;67(1):94–8.
-
Hu X, Fang Y, Ye F, Lin S, Li H, You C, et al. Effects of plasma D-dimer levels on early mortality and long-term functional outcome after spontaneous intracerebral hemorrhage. J Clin Neurosci. 2014;21(8):1364–7.
-
Miskowiak K, Johnsen S, Sattler S, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39–48.
-
Saand AR, Yu F, Chen J, Chou SH-Y. Systemic inflammation in hemorrhagic strokes – A novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. 2019;39(6):959–88.
-
Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-Lymphocyte Ratio Predicts the Outcome of Acute Intracerebral Hemorrhage. Stroke. 2016;47(6):1654–7.
-
Khandekar MM. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006;59(2):146–9.
-
Mutzenbach JS, Müller-Thies-Broussalis E, Killer-Oberpfalzer M, Griessenauer CJ, Hecker C, Moscote-Salazar LR, et al. Severe Leukoaraiosis Is Associated with Poor Outcome after Successful Recanalization of M1 Middle Cerebral Artery Occlusion Strokes. Cerebrovasc Dis. 2020;49(3):253–61.
Articole din ediţiile anterioare
Modele de adaptare şi acceptare a schimbării la femeile din România. Resurse, limite şi consecinţe
Obiectivul principal al lucrării de faţă este prezentarea mecanismelor de coping folosite de femeile care se consideră afectate de criza economică ...
Reabilitarea psihosocială: un mit sau un domeniu legitim în psihiatria modernă?
Tulburările psihice severe reprezintă una din principalele cauze de dizabilitate la nivel mondial. Asocierea stigmei conduce invariabil la scăderea...
Analiza transnosografică a disfuncţiei executive – dimensiuni clinice, psihometrice şi terapeutice (II)
Deficitele la nivelul planificării, atenţiei selective, iniţierii activităţilor, controlului interferenţelor şi inhibiţiei răspunsului, diminuarea ...
Trăsături personologice individuale în opţiunea profesională a studenţilor medicinişti şi a medicilor rezidenţi
Specialitatea medicală contribuie la procesul de formare profesională, prin dezvoltarea competenţelor personale, prin acumularea permanentă de cuno...