Clozapine-resistant or ultra-resistant schizophrenia represents a challenge for clinicians, given the lack of recommendations in all the major treatment guidelines regarding the next steps once clozapine has failed or cannot be tolerated. The first part of this narrative review investigated the pharmacological arsenal available to the clinician to deal with cases of clozapine-resistant schizophrenia. In the present section of the analysis, the results of research that explored the efficacy and tolerability of nonpharmacological methods for this type of schizophrenia will be presented. Ongoing or upcoming clinical trials in patients with ultra-resistant schizophrenia will also be reviewed. Searching electronic databases and clinical trial archives identified seven reports on the effects of psychotherapy and 15 reports on neuromodulation techniques that aimed at ameliorating psychotic symptoms and functionality in clozapine-resistant schizophrenia. A number of six ongoing clinical trials targeting this type of schizophrenia are also presented. In conclusion, based on the data analyzed, cognitive-behavioral therapy for psychosis, cognitive-behavioral therapy, and occupational therapy benefit from moderate-quality data to support their efficacy as add-ons to clozapine in these patients. Except for electroconvulsive therapy, the evidence for other neuromodulation techniques is, at least for now, insufficient to recommend them in ultra-resistant cases. It is necessary to carry out good quality and long-term clinical studies in order to confirm the usefulness of these adjuvant treatments.
Therapeutic options in ultra‑resistant schizophrenia. Nonpharmacological interventions (II)
Opţiuni terapeutice în schizofrenia ultrarezistentă. Intervenţii nonfarmacologice (II)
First published: 30 iunie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Psih.73.2.2023.8254
Abstract
Rezumat
Schizofrenia rezistentă la clozapină, sau schizofrenia ultrarezistentă, reprezintă o provocare pentru clinicieni, dată fiind lipsa de recomandări din ghidurile terapeutice privind paşii următori odată ce clozapina nu a condus la efectele scontate. În prima parte a acestei analize narative a fost investigat arsenalul farmacologic pe care clinicianul îl are la dispoziţie pentru a face faţă cazurilor de schizofrenie rezistentă la clozapină. În această parte a analizei vor fi prezentate rezultatele cercetărilor care au explorat eficacitatea şi tolerabilitatea metodelor nefarmacologice destinate acestei forme de schizofrenie. De asemenea, vor fi trecute în revistă studiile clinice aflate în desfăşurare sau care urmează a începe, dedicate pacienţilor cu schizofrenie ultrarezistentă. În urma căutării bazelor de date electronice şi a arhivelor de studii clinice, au fost identificate şapte surse privind efectele psihoterapiei şi 15 surse referitoare la tehnicile de neuromodulare având ca scop ameliorarea simptomatologiei în schizofrenia rezistentă la clozapină. Un număr de şase studii clinice aflate în desfăşurare şi care vizează această formă de schizofrenie au fost, de asemenea, prezentate. În concluzie, pe baza datelor analizate, terapia cognitiv-comportamentală pentru psihoze, terapia cognitiv-comportamentală şi terapia ocupaţională dispun de date de calitate moderată privind eficienţa asocierii lor cu clozapină în cazul acestor pacienţi. Cu excepţia terapiei electroconvulsivante, dovezile referitoare la alte tehnici de neuromodulare sunt, cel puţin deocamdată, insuficiente pentru a le putea recomanda de rutină în aceste cazuri. Pornind de la rezultatele prezentate, reiese că este nevoie de realizarea unor studii clinice de bună calitate şi pe durate îndelungate pentru a putea confirma utilitatea acestor intervenţii adjuvante.
From pharmacological to nonpharmacological interventions in clozapine-resistant schizophrenia
The necessity to investigate clozapine-resistant schizophrenia (CRS) cannot be overemphasized due to the significant negative impact this pathology has on patients’ daily functioning, quality of life, and general prognosis(1).
Completing the data presented in the first part of this narrative review, when combining clozapine with different pharmacological agents, it is important to consider their receptoral binding profile and pharmacokinetic properties, in order to increase the treatment’s efficacy and decrease the risk of adverse events. This is an essential aspect because: (1) multiple ligands acting on the same receptors may compete for those targets, or may enhance each other’s adverse effects, causing low efficacy and reduced tolerability, respectively; (2) a certain degree of D2 receptor occupancy in the striatum is needed for the onset of the antipsychotic effect (60-78%), but an increase of the D2 occupancy to more than 80% was associated with a significant risk of adverse events (e.g., extrapyramidal symptoms, hyperprolactinemia, cognitive impairment, etc.)(2,3); (3) caution is needed when combining partial agonists with antagonists for the same receptors (e.g., D2 receptors); (4) not all agents could be adequately monitored using biological variables (e.g., plasma levels), therefore knowing potential pharmacokinetic interactions at CYP450 or P-glycoprotein system may be helpful in anticipating low efficacy or higher risk of adverse events; (5) pharmacogenetic profiling may be necessary in case of therapeutic resistance, or when tolerability is reduced, although low doses of antipsychotics are administered.
Starting from the aforementioned cautionary statements, the clinician needs to know that clozapine has a specific pharmacodynamic profile, partially explaining its unique properties in treatment-resistant schizophrenia. This antipsychotic presents a high affinity for dopamine D4 receptors, leading to a low rate of extrapyramidal symptoms compared to other antipsychotics with high D2 receptor potency(4). Clozapine possesses 5HT1A receptor partial agonistic properties, and M1, M2, M3, M5, histamine and adrenergic alpha-1 receptor antagonistic properties(4). Unlike clozapine and other second-generation agents, first-generation antipsychotics antagonize D2 receptors while having significant noradrenergic, cholinergic and/or histaminergic blocking properties(5).
Like clozapine, quetiapine is a weak D2 antagonist, while risperidone, ziprasidone, olanzapine and paliperidone are potent D2 ligands(6). Aripiprazole, brexpiprazole and cariprazine are D2 receptors partial agonists, and they are considered “third-generation antipsychotics”. Aripiprazole has the highest intrinsic activity at D2 receptors and cariprazine has a high selectivity for D3 receptors, therefore the first agent may be activating, while the second may decrease positive, negative and cognitive symptoms(7). Sertindole exerts a potent antagonism at D2, 5HT2A, 5HT2C and alpha-1 adrenergic receptors, with low affinity for M1 and H1 receptors(8). Amisulpride binds at high doses preferentially to postsynaptic D2/D3 receptors, with antagonistic properties, while at low doses it blocks presynaptic D2/D3 receptors and enhances dopamine transmission(9). Sulpiride is a first-generation antipsychotic with selective D2/D3 receptor antagonism, while loxapine has a high affinity for postsynaptic D2/D3 receptors and 5HT2A receptors, being considered a “typical antipsychotic with atypical properties” (10).
All these pharmacodynamic properties, together with the pharmacokinetic characteristics of each agent, should be considered especially when (1) a combination of antipsychotics is used; (2) an antipsychotic is discontinued, or a switch is intended; (3) adverse events are observed, and could not be explained by the prescribed doses of antipsychotics; (4) the efficacy is lower than expected in case two agents are combined; (5) multiple medications are administered concomitantly with the antipsychotic(s), for different comorbid conditions.
Based on the reviewed reports in the first part of this article, clozapine augmentation with another antipsychotic, which possesses a complementary pharmacodynamic profile, may be useful if the presence of CRS is certain and all other therapeutic interventions have been exhausted; appropriate monitoring of treatment efficacy and adverse events is necessary throughout the trial of combined pharmacological agents; the shortest duration possible of the combined treatment is also recommended(11,12). Also, an atypical antipsychotic with a long-acting injectable formulation (evidence for aripiprazole monohydrate and risperidone microspheres exists) may be recommended, especially where treatment adherence is problematic(13,14). These augmenting strategies may be useful mainly for persistent positive symptoms during clozapine administration. Monitoring the plasma concentration of clozapine is also strongly recommended, and the daily dose of clozapine may be reduced as a consequence of introducing the second antipsychotic. Data about the efficacy of switching from clozapine to other antipsychotic(s) is sparse, but oral olanzapine or amisulpride, and long-acting injectable antipsychotics (i.e., aripiprazole, paliperidone) could be useful(15-17). No good quality data have been obtained about the efficacy of combining two antipsychotics, other than clozapine, or about the administration of higher doses of atypical antipsychotics in the case of CRS. The reinitiation of clozapine could be recommended if no severe adverse events were reported during the first trial(18,19).
Mood stabilizers – especially topiramate, lamotrigine and sodium valproate – may be added to clozapine for increased efficacy, but the data are not sufficient to formulate a specific regimen (dose, duration, monitoring methods etc.)(20-26). Certain antidepressants (mainly selective serotonin reuptake inhibitors [SSRIs], mirtazapine and duloxetine) have been added to clozapine for negative symptoms with favorable results(27-32); however, data are derived only from case reports and small-scale trials, therefore caution is recommended. Other agents (benzodiazepine, memantine, donepezil etc.) are still far from being supported by solid evidence for their use in CRS patients(33,34).
Other sources support similar conclusions. According to a guideline edited by the Treatment Response and Resistance in Psychosis (TRIPP) Working Group(35), waiting for a response to clozapine was the first recommendation in case of positive symptoms, when clozapine plasma levels were ≥350 ng/ml. If positive symptoms are resistant to clozapine, then amisulpride or oral aripiprazole may be recommended as add-ons; augmentation with electroconvulsive therapy (ECT) could also be initiated(35). For clozapine-resistant negative symptoms, the addition of an antidepressant is recommended(35). In the case of clozapine-resistant suicidality, antidepressants, mood stabilizers or ECT may be administered, and for aggressivity, a mood stabilizer or antipsychotic may be initiated as add-ons to clozapine(35).
Other pharmacological agents and combinations of such agents are explored in schizophrenia (e.g., cannabinoids, antibiotics, anti-inflammatory agents etc.), but no specific reference to CRS could be found in literature(36,37). The investigation of possible causes for therapeutic nonresponsivity should constitute a priority for further research in the domain of CRS(38). This includes pharmacogenetic analysis for the detection of treatment resistance predictors. Screening for comorbidities – especially for substance use disorders – could be helpful in all cases of CRS(38). The addition of psychosocial interventions or neuromodulatory techniques should be taken into consideration in these patients(39), and these strategies are investigated in the second part of the review.
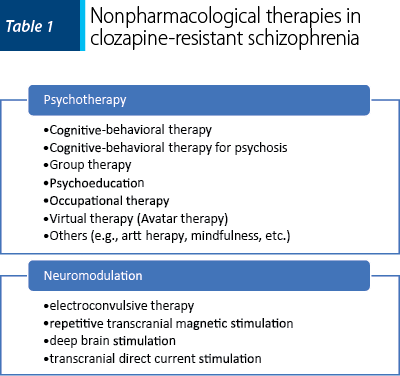
Psychotherapy has been explored in patients with schizophrenia as an add-on to the pharmacologic intervention(40). Of the psychotherapies investigated for this specific population, cognitive-behavioral therapy (CBT) has the most evidence to support its recommendation(40). Reduction of residual symptoms in outpatients with schizophrenia has been demonstrated for CBT, but other interventions can also be beneficial – i.e., compliance therapy, acceptance and commitment therapy, and supportive therapy(40). Newer psychotherapeutic approaches are also explored, but data to prove their efficacy are still pending – i.e., narrative therapy, metacognitive therapy, cognitive enhancement therapy, and mindfulness(40,41). Cognitive-behavioral therapy for psychosis (CBTp) is a specific type of therapy that includes particularities regarding the engagement of the patient (e.g., reducing the stigma by normalization, client feedback is essential, expression of positive and negative reaction toward therapy is encouraged, etc.), structure and principles (e.g., careful explanations, reducing distress, elicitation of hope in recovery, agreeing on a problem list, practical plans, etc.), and change strategies (e.g., self-disclosure is allowed, exploration of hallucinations, direct working with the content of the auditory hallucinations, etc.)(42).
Occupational therapy is based on the need to engage patients with schizophrenia in meaningful activities which increase their self-esteem, feeling of accomplishment, general well-being, and tendency toward social inclusion(43). Expressive art, crafts and group-oriented recreational activities are meant to improve these patients’ self-esteem and functionality(43,44). In patients with chronic schizophrenia, occupational therapy (group intervention; 18 hours per week) combined with medication can improve the total scores of the Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms (SAPS) at six months versus the control group(43).
Family interventions for patients with schizophrenia include psychoeducation, techniques focused on stress reduction and emotional processing, structured problem solving, and guided cognitive reinterpretation(45). The main purpose of this approach is to develop a collaborative relationship between the family members, the therapeutic team and the patient(44). Family therapy has been associated in clinical trials with favorable effects on the patient’s ability to recover from acute psychotic episodes and with improvements in their social functioning(45,46).
Psychodynamic psychotherapy could bring some benefits to patients with schizophrenia and severe mental disorders in the short term, but in the long term, no clear favorable effect has been supported by evidence(47). A Cochrane review of trials dedicated to this topic (n=4, N=528 participants) reported that the use of psychodynamic psychotherapy was associated with lower use of medication, but it did not influence the rehospitalization rate and, even more, it negatively impacted the discharge rate(48).
Neuromodulation techniques have been explored for patients with schizophrenia as add-ons to pharmacological treatment when partial efficacy or lack of tolerance for certain medications was detected. Neuromodulation interventions explored for this population include deep brain stimulation (DBS), and transcranial magnetic and electrical stimulation(49). This type of treatment is based on the data derived from human trials and preclinical studies that identified certain neurobiological dysfunctions in major psychiatric disorders(49). It should be noted, however, that the complexity of psychiatric disorders, schizophrenia spectrum disorders included, prevents the possibility of identifying specific areas that, if stimulated, will have the same degree of impact as it is the case with several neurological diseases – e.g., targeting the subthalamic nucleus in Parkinson disease with DBS(50).
Objective and method
The main objective of this review was to identify the available evidence to support the efficacy and safety of psychotherapy and neuromodulation intervention in patients with CRS. The secondary objective was to find trials that are ongoing or planned to begin in the near future dedicated to this type of schizophrenia. The details about the methodology were mentioned in the first part of the review.
Results
After the search paradigm was applied and the results were filtered according to the objective of the current review, sources were distributed according to the main intervention they assessed. One case report, three clinical trials/retrospective studies, and two reviews/meta-analyses were found for psychotherapy. Regarding the neuromodulation techniques, seven clinical trials, three case reports/case series, and five reviews/meta-analyses were found. Six clinical trials are ongoing or planned to begin soon, all of them assessing diverse interventions for patients with CRS.
Psychotherapy added to clozapine
Although only a few data exist about the efficacy of psychotherapy on patients with clozapine-resistant schizophrenia, psychosocial interventions are commonly added in clinical practice – i.e., adherence enhancement therapy, familial therapy, occupational therapy etc. A systematic review (n=42 articles) concluded that cognitive-behavioral therapy (CBT) is the most frequent psychotherapy added to the pharmacological regimen in treatment-resistant schizophrenia, reaching 76% of all the interventions explored(51). CBT was associated with efficacy for general psychopathology and positive symptoms, but had an uncertain efficacy for negative symptoms(51). Group therapy was efficient in studies that investigated this type of intervention(51).
Cognitive remediation, supportive counseling, psychoeducation, social skills training, individual multimodal psychotherapy, mindfulness, art group therapy, occupational therapy, metacognitive therapy and psychodynamic interpersonal therapy were also explored, but with less evidence in their favor(51). However, in 40 of the 42 studies included in this review, psychotherapy demonstrated improvement in at least one clinical outcome(51).
Occupational therapy added to clozapine has proven its efficacy in patients with treatment-resistant schizophrenia in a randomized controlled trial (N=26 participants) versus clozapine monotherapy(52). The Scale for Interactive Observation in Occupational Therapy scores began to improve mainly after 16 weeks of occupational therapy in patients with combined treatment (pharmacologic + psychosocial), and the improvement was observed throughout the duration of the study(52).
In patients with clozapine resistance, the addition of CBT for psychosis (CBTp) may lead to benefits for persistent positive symptoms, but the effect size is small (n=4 trials)(53). The benefits were detected at the endpoint, but also at six and 12 months of follow-up(53).
In a randomized controlled, assessor-blinded trial, CBT was added to clozapine in patients with schizophrenia who had persistent psychotic symptoms (N=487 participants) and compared to treatment as usual(54). No difference was detected in the primary outcome (i.e., Positive and Negative Syndrome Scale [PANSS] total score at month 21), but CBT improved this outcome after nine months (i.e., the end-point), although not significantly(54). There was no indication that CBT may be correlated with potential adverse events(54). Based on these results regarding CBT’s efficacy, the authors considered that this therapy should not be routinely offered in clozapine-resistant patients.
In patients with the first episode of psychosis who had an unfavorable evolution (N=48 participants), a randomized trial evaluated the efficacy of four interventions: clozapine, clozapine + CBT, thioridazine, and thioridazine + CBT during 12 weeks(55). At the end-point, all groups presented with improvements for the outcomes measured: Brief Psychiatric Rating Scale (BPRS), SANS, Beck Depression Inventory (BDI), Clinical Global Impression (CGI), and Social and Occupational Functioning Assessment Scale (SOFAS)(55). Clozapine was superior to thioridazine for positive symptoms, but the addition of CBT to thioridazine reduced the difference when compared to clozapine as monotherapy(55). Negative symptoms were reduced more when CBT was added to either antipsychotic, with almost 65% compared to baseline values(55). The functionality improved in all groups, but the effect size was modest and there was no significant difference between interventions(55). CBT was considered to stabilize symptoms in the medium term (up to six months)(55).
Avatar therapy (AvT) is a new experiential intervention based on the possibility of each patient creating an avatar of their persecutor and focusing on gaining control over their symptoms(56). A case report presents the effects of AvT in a patient with ultra-resistant schizophrenia, who also tried CBT and rTMS (repetitive transcranial magnetic stimulation) before, but with little to no benefit(56). The favorable impact of seven weekly sessions of AvT was detected especially on positive symptoms and depressive manifestations, and the core symptom (auditory hallucinations) disappeared (according to the Psychotic Symptoms Rating Scale [PSYRATS], PANSS, and BDI-II)(56). As a consequence, his functionality improved significantly(56). However, it should be noted that this is just a case report, and more research is needed before the validation of AvT in this specific population.
Neuromodulation techniques as an add-on to clozapine
A review of trials dedicated to the combined electroconvulsive therapy (ECT) and clozapine treatment (n=40, N=208 participants diagnosed with schizophrenia or schizoaffective disorder) concluded that between 37% and 100% of these patients improved in the short term(57). The long-term improvement (up to 24 months) was reported only in a few studies(57). Delirium and tachycardia, but also prolonged seizures were reported as adverse events (n=14 patients, in total)(57).
The administration of ECT in CRS has been associated with high relapse rates (up to 44%) in the first six months after the discontinuation of this therapeutic intervention in patients with major psychiatric illnesses(58). A randomized controlled trial, single-blinded, designed to enroll 64 adult participants presenting CRS, is expected to evaluate the effects of short-term and long-term ECT prospectively(59,60). This trial has as its main outcome measures the response rate (i.e., a 30% decrease in the BPRS rate) at the 15th month – i.e., at three months after the end of the treatment(59,60).
A prospective study that evaluated the effects of various add-on strategies to clozapine (i.e., ECT sessions for undifferentiated schizophrenia, amisulpride or aripiprazole for disorganized schizophrenia, and risperidone or ECT for paranoid cases), in ten patients with CRS, reported improvements of BPRS scores between 34% and 40%(61).
A randomized, single-blind, eight-week trial enrolled 39 participants with CRS who received either bilateral ECT plus clozapine or clozapine as the only active intervention during eight weeks(62). At the end of the study, no significant difference was detected between the two groups on global cognition, but patients treated with ECT had a 50% remission rate versus none in the control group(62). The ECT intervention was safe throughout the study(62). However, this was a short-term study that needed to be completed with follow-up visits. Another trial explored the efficacy of continuation ECT in patients with CRS who completed the acute study and responded to this intervention (N=19)(63). The continuation regimen consisted of ten ECT sessions over six months, and the completers (N=14) showed a favorable response, according to the BPRS scores(63). Also, none of the participants presented relevant worsening symptoms(63). It is worth mentioning that this was only a pilot study, and larger trials are needed to support this conclusion. In a retrospective analysis, 42 patients with URS received clozapine plus ECT (the mean number of ECTs was 10.6, with a range of 3-25), and 76% of cases reported increases in their CGI-Improvement (CGI-I) scores(64). The tolerability was good, and in 64% of participants there were observed no adverse events(64).
rTMS is a neuromodulation intervention explored in many psychiatric disorders, including ultra-resistant schizophrenia. In an open-label retrospective study, patients with treatment-resistant auditory verbal hallucination in the context of schizophrenia (N=14, including nine on clozapine) received 30 sessions of rTMS on the temporoparietal junction during three weeks(65). A significant decrease in positive symptoms was detected after the low-frequency rTMS application, according to the Auditory Hallucination Rating Scale (AHRS)(65). This favorable evolution was also observed in the nine patients who received clozapine + rTMS(65).
However, a review of ten randomized, controlled trials that explored the effects of rTMS in patients with schizophrenia who received clozapine (N=131) did not support the existence of a significant impact (response rates) in the domains of auditory hallucinations, negative symptoms or general psychopathology between sham and rTMS groups(66). The number needed to treat (NNT) for rTMS was nine for auditory hallucinations, which still indicates a possible utility for this intervention(66). The impact on PANSS scores also was not significant in sham versus rTMS(66).
Another systematic review and pair-wise meta-analysis (n=24 studies; three for meta-analysis) dedicated to the augmentation of clozapine with rTMS concluded that there is no difference between sham and rTMS for total, positive and negative symptoms(67). There were differences in the location of the rTMS electrode between studies and, also, the type of sham varied, therefore a strict comparison is difficult to make due to the heterogeneity of the methodology used(67).
Certain individual studies contradict these results. In a trial with 26 patients with ultra-resistant schizophrenia who were receiving clozapine, an rTMS versus sham (five sessions/week, three weeks) comparison showed that the active intervention led to a significant decrease in the PANSS positive subscale and PANSS total scale scores(68). No effect of rTMS was detected on the PANSS negative subscale at the endpoint (day 105)(68).
Deep brain stimulation (DBS) has also been used in treatment-resistant psychiatric disorders, but in refractory schizophrenia it is less explored. One possible explanation is the uncertainty about the placement of electrodes(69,70). According to several authors, the zones of interest for DBS would be the nucleus accumbens and subgenual anterior cingulate cortex, while the preferred measure for clinical improvement remains PANSS assessed fortnightly(69). Other authors suggest the ventral tegmental area, substantia nigra pars reticulata, and the habenula as areas of interest for DBS in treatment-resistant schizophrenia(70).
In seven patients with schizophrenia who could not tolerate or were resistant to clozapine, DBS was successful in achieving stabilization (PANSS total score decreased by more than 25% versus baseline) after 24 weeks(69). Patients who improve during DBS tend to worsen when entering the crossover phase(69). There were reported very few physical adverse events, but in two cases persistent psychiatric adverse events were noted (i.e., negative symptoms and mood instability)(69).
A review that included eight human trials and two preclinical studies exploring DBS (targeting the nucleus accumbens, subgenual anterior cingulate cortex, habenula, and substantia nigra pars reticulata) and neurosurgical interventions (i.e., subcaudal tractotomies and anterior capsulotomies) for schizophrenia concluded there are conflicting results(71). However, the majority of the human trials reported long-term reductions in the PANSSS scores, with a reduced rate of psychological adverse events(71).
Transcranial direct current stimulation (tDCS) was explored in patients with persistent or severe hallucinatory manifestations in schizophrenia, which are treatment-resistant, including to clozapine administration(72). The placement of the cathode over the left temporoparietal cortex and the anode on the left dorsolateral prefrontal cortex has been suggested, with once or twice daily sessions(44). The data to support tDCS for this indication are limited, with one randomized trial and several case reports showing benefits for up to three years(72). The tolerability of tDCS was good for long-term monitoring periods(72).
In a case report, the administration of once daily, 20-minute tDCS sessions produced improvement in cognitive and psychosocial functioning, followed by a decrease in the severity of the hallucinations, in a 24-year-old female patient with clozapine-refractory schizophrenia(73). The tDCS was applied with an increase in amperage, frequency and duration of sessions after two months, which led to further improvements in psychosocial functioning(73). After three years of tDCS sessions, benefits persisted and no adverse events were reported(73).
In another case report, tDCS not only improved residual persistent auditory hallucinations in a 47-year-old male diagnosed with treatment-resistant schizophrenia, but also reduced the clozapine-induced severe hypersalivation(74). This patient received 450 mg of clozapine daily, augmented with haloperidol, but the hallucinations persisted until 15 sessions of tDCS targeting the left temporoparietal junction were applied, leading to a significant decrease in the PANSS score(74).
Trials in the pipeline
The investigation of ECT in patients with ultra-resistant schizophrenia is intended to be explored in a multicentric, prospective, randomized, controlled study, as an add-on to clozapine in 64 patients(75). The monitoring period is six weeks, and bilateral ECT will be applied twice a week for the first six weeks and once a week for another four weeks; in the third phase, patients will receive one ECT each month for two months(75). The second arm of this study will imply bilateral ECT twice a week for 12 weeks, once a week for eight weeks, and one ECT session every three weeks for another 16 weeks(75). The main outcome is the response rate (over 30% decrease in the PANSS total scores) in the 15th month(75).
A sequential multiple-stage randomized controlled phase III trial will recruit 162 people with treatment-resistant schizophrenia and monitor them for 12 months(76). The interventions to be compared with are clozapine (400-600 mg/day), clozapine + amisulpride (400-600 mg/day and 200-800 mg/day, respectively), clozapine + Ginkgo biloba (400-600 mg/day and 120-360 mg/day, respectively), modified ECT (16 sessions during four months), multisystemic therapy (MST, 16 sessions for four months) and DBS (16 sessions during four months), and the main outcome is the response rate (defined by PANSS total score change ≥25% at week 12)(76).
DBS is the main intervention in an open-label pilot trial expected to enroll three participants with treatment-resistant schizophrenia who will receive stimulation of the substantia nigra pars reticulata(77). The main outcomes are changes in the SANS, BPRS and incidence of adverse events related to the device used one year after the DBS application(77).
A phase II trial is expected to enroll 80 participants diagnosed with ultra-resistant schizophrenia in order to evaluate the effects of an N-methyl-D-aspartate (NMDA)-enhancer to anti-inflammatory treatments versus NMDA-enhancer plus placebo during 12 weeks(78). The primary outcome measures will be the change in cognitive function after three months, using specific tests for the assessment of seven cognitive domains (i.e., speed of processing, sustained attention, working memory, verbal learning and memory, visual learning and memory, reasoning and problem solving, and social cognition)(78).
A phase II/III double-blind, randomized, placebo-controlled multicenter study is designed to explore the efficacy and safety of sodium benzoate (NaBen®) as an add-on to clozapine in 287 patients with refractory schizophrenia(79). The primary outcome of this study is the mean change from baseline in PANSS total scores at week 8 after randomization(79). This investigational product is an NMDA-receptor enhancer by inhibiting the degradation of D-serine by D-amino acid oxidase, explored mainly for the improvement of negative and cognitive symptoms of schizophrenia, but also for positive manifestations of psychosis(80).
Minocycline is investigated as an augmentation of clozapine in treatment-resistant schizophrenia in a phase I trial planned to enroll 60 participants randomized on 200 mg/day minocycline or placebo for 12 weeks(81). The main outcome is the change in PANSS total score at week 12(81).
Conclusions
As a cautionary note, in clinical practice, it is essential to explore the available, evidence-based therapeutic interventions in patients with CRS only after ensuring the diagnosis was correctly made, by eliminating potential confounders – e.g., organic and/or psychiatric comorbidities, substance use disorders, pharmacogenetic factors, potential negative pharmacokinetic interactions, clozapine plasma levels etc.(82)
Psychotherapy – especially CBT, CBTp and occupational therapy – benefits from multiple evidence to support their use as add-ons to pharmacological therapy in CRS(51-55). Based on current evidence, CBT could be recommended for managing general psychopathology and positive symptoms in CRS patients, but it had an uncertain efficacy for negative manifestations(51). Third-generation CBT could also be investigated for this indication, but until now there is no clear evidence to recommend them(83).
Neuromodulation techniques have been explored, and new trials are ongoing, with the objective of strengthening the level of evidence for these interventions in patients with CRS(58-77). Except for ECT, the data to support rTMS are sparse, with a certain level of contradictory evidence, while DBS and tDCS are still in earlier phases of research for CRS. The efficacy of ECT was demonstrated in the short term, but trials of good quality to support its efficacy in the long term still need to be developed, and a high rate of relapse has also been reported (see Lambrichts et al.(58), for example).
New drugs are currently undergoing investigation, but older pharmacological agents could also gain new recommendations through clinical trials(76,78-81).
The limitations of this review refer to its narrative methodology, therefore no well-defined inclusion and exclusion criteria for the explored reports were predefined. Also, it should be noted that the quality of the reviewed reports is quite heterogenous, but most of them are of medium quality and based on small samples. However, good quality primary and secondary reports are included, such as randomized clinical trials and meta-analyses. The heterogeneity of therapeutic investigations explored here (methodological differences between various CBT programs or different placement of electrodes for neuromodulatory therapies, for example) could impact the level of evidence to support clinical recommendations. The need for further exploring the efficacy and tolerability of pharmacological interventions for CRS is acute, as the negative consequences of CRS over the daily functioning of these patients are significant.
In conclusion, more good quality clinical trials dedicated to patients with CRS are needed, especially with long-term follow-ups. Because some therapeutic interventions presented here are still experimental or relatively new for CRS patients, it is expected that future research may significantly change their level of recommendation.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.

Bibliografie
- Campana M, Falkai P, Siskind A, Wagner E. Characteristics and definitions of ultra-treatment-resistant schizophrenia - A systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
- de Greef R, Maloney A, Olsson-Gisleskog P, Schoemaker J, Panagides J. Dopamine D2 occupancy as a biomarker for antipsychotics: quantifying the relationship with efficacy and extrapyramidal symptoms. AAPS J. 2011;13(1):121-30.
- Sakurai H, Bies RR, Stroup ST, Keefe ESE, Rajji TK, Suzuki T, et al. Dopamine D2 receptor occupancy and cognitive in schizophrenia: Analysis of the CATIE data. Schizophr Bull. 2013;39(3):564-574.
- Haidary HA, Padhy RK. Clozapine. StatPearls (Internet). Treasure Island, FL, Stat Pearls Publishing, 2022. Accessed online at https://www.ncbi.nlm.nih.gov/books/NBK535399/. Retrieved 06 Nov 2022.
- Chockhawala K, Stevens L. Antipsychotic medications. Treasure Island, FL, Stat Pearls Publishing, 2022. Accessed online at https://www.ncbi.nlm.nih.gov/books/NBK519503/. Retrieved 06 Nov 2022.
- Wilmer K, Vasan S, Abdijadis S. Atypical antipsychotic agents. StatPearls (Internet). Treasure Island, FL, Stat Pearls Publishing, 2022. Accessed online at https://www.ncbi.nlm.nih.gov/books/NBK535399/. Retrieved 06 Nov 2022.
- Mohr P, Masopust J, Kopecek M. Dopamine receptor partial agonists: Do they differ in their clinical efficacy? Front Psychiatry. 2021;12:781946.
- Juruena MF, de Sena EP, de Oliveira IR. Sertindole in the management of schizophrenia. J Cent Nerv Syst Dis. 2011;3:75-85.
- McKeage K, Plosker GL. Amisulpride: a review of its use in the management of schizophrenia. CNS Drugs. 2004;18(13):933-56.
- Popovic D, Nuss P, Vieta E. Revisiting loxapine: a systematic review. Annals General Psychiatry. 2015;14:15.
- Barber S, Olotu U, Corsi M, Cipriani A. Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2017;3(3):CD006324.
- Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology.
- J Psychopharmacol. 2020;34(1):3-78.
- Kim SH, Jung DC, Ahn YM, Kim YS. The combined use of risperidone long-acting injection and clozapine in patients with schizophrenia non-adherent to clozapine: a case series. J Psychopharmacol. 2010;24(7):981-6.
- Sepede G, Di Iorio G, Spano MC, Lorusso M, Sarchione F, Santacroce R, et al. A case of resistant schizophrenia successfully treated with clozapine/long-acting injectable aripiprazole combination. Clin Neuropharmacol. 2016;39(6):322-324.
- Pehlivanidis A, Spyropoulou AC, Tourkantonis A, Papadimitriou GN. Amisulpride monotherapy in a patient with clozapine-resistant schizophrenia. Clin Neuropharmacol. 2010;33(3):168.
- Dossenbach NRK, Beuzen JN, Avnon M, Belmaker RH, Elizur A, Mark M,
- et al. The effectiveness of olanzapine in treatment-refractory schizophrenia when patients are nonresponsive to or unable to tolerate clozapine. Clin Ther. 2000;22(9):1021-34.
- Martínez-Andrés JA, García-Carmona JA. Switching from clozapine to paliperidone palmitate-3-monthly improved obesity, hyperglycemia and dyslipidemia lowering antipsychotic dose equivalents in a treatment-resistant schizophrenia cohort. Int Clin Psychopharmacol. 2020;35(3):163-169.
- Stam N, Taipale H, Tanskanen A, Isphording L, Okhuijsen-Pfeifer C, Schuiling-Veninga CCM, et al. Persistence of antipsychotic use after clozapine discontinuation: A real-world study across antipsychotics. Clin Transl Sci. 2020;13(6):1170-1177.
- Luykx JJ, Stam N, Tanskanen A, Tiihonen J, Taipale H. In the aftermath of clozapine discontinuation: comparative effectiveness and safety of antipsychotics in patients with schizophrenia who discontinue clozapine.
- Br J Psychiatry. 2020;217(3):498-505.
- Zheng W, Xiang YT, Yang XH, Xiang YQ, de Leon J. Clozapine augmentation with antiepileptic drugs for treatment-resistant schizophrenia: A meta-analysis of randomized controlled trials. J Clin Psychiatry. 2017;78(5):e498-e505.
- Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-4.
- Small JG, Klapper MH, Malloy FW, Steadman TM. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol. 2003;23(3):223-8.
- Hahn MK, Remington G, Bois D, Cohn T. Topiramate augmentation in clozapine-treated patients with schizophrenia: clinical and metabolic effects.
- J Clin Psychopharmacol. 2010;30(6):706-10.
- Muscatello MRA, Bruno A, Pandolfo G, Mico U, Bellinghieri PM, Scimeca G, et al. Topiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. J Psychopharmacology. 2011;25(5):667-74.
- Kelly DL, Conley RR, Feldman S, Yu Y, McMahon RP, Richardson CM. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatry Q. 2006;77(1):81-95.
- Demily C, Franck N. Gabapentin for ultra resistant schizophrenia with aggressive behavior. Schizophr Res. 2008;100(1-3):349-50.
- Edinoff AN, Fort JM, Woo JJ, Causey CD, Burroughs CR, Cornett EM, Kaye AM, Kaye AD. Selective serotonin reuptake inhibitors and clozapine: Clinically relevant interactions and considerations. Neurol Int. 2021;13(3):445-463.
- Szegedi A, Anghelescu I, Wiesner J, Schlegel S, Weigmann H, Härtter S,
- et al. Addition of low-dose fluvoxamine to low-dose clozapine monotherapy in schizophrenia: drug monitoring and tolerability data from a prospective clinical trial. Pharmacopsychiatry. 1999;32(4):148-54.
- Strous RD, Patel JK, Zimmet S, Green AI. Clozapine and paroxetine in the treatment of schizophrenia with obsessive-compulsive features. Am J Psychiatry. 1999;156(6):973a-974.
- Buchanan RW, Kirkpatrick B, Bryant N, Ball P, Breier A. Fluoxetine augmentation of clozapine treatment in patients with schizophrenia. Am J Psychiatry. 1996;153(12):1625-7.
- Zoccali R, Muscatello MR, Cedro C, Neri P, la Torre D, Spina E, et al. The effect of mirtazapine augmentation of clozapine in the treatment of negative symptoms of schizophrenia: a double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2004;19(2):71-6.
- Mico U, Bruno A, Pandolfo G, Romeo VM, Mallamace D, Dárrigo C, et al. Duloxetine as adjunctive treatment to clozapine in patients with schizophrenia: a randomized, placebo-controlled trial. Int Clin Psychopharmacol. 2011;26(6):303-10.
- Szarmach J, Wlodarczyk A, Cubala WJ, Wiglusz MS. Benzodiazepines as adjunctive therapy in treatment refractory symptoms of schizophrenia. Psychiatria Danubiana. 2017;29(Suppl.3):349-352.
- Grohmann R, Rüther E, Sassim N, Schmidt LG. Adverse effects of clozapine. Psychopharmacol (Berl). 1989;99(Suppl.):S101-4.
- Wagner E, Kane JM, Correll CU, Howes O, Siskind D, Honer WG, et al. Clozapine combination and augmentation strategies in patients with schizophrenia - Recommendations from an international expert survey among the treatment response and resistance in psychosis (TRIPP) working group. Schizophr Bull. 2020;46(6):1459-1470.
- Vasiliu O, Vasile D, Voicu V. Efficacy and tolerability of antibiotic augmentation in schizophrenia spectrum disorders - A systematic literature review. RJMM. 2020;CXXIII(1):3-20.
- Vasiliu O, Vasile D, Făinărea AF, Pătraşcu MC, Morariu EA, Manolache R, Alexandru I, Androne FT. Analysis of risk factors for antipsychotic-resistant schizophrenia in young patients- a retrospective analysis. RJMM. 2018;CXXI(1):25-29.
- Vasiliu O. Therapeutic management of schizophrenia and substance use disorders dual diagnosis - clinical vignettes. RJMM. 2018;CXXI(2):26-34.
- Dokucu ME. Neuromodulation treatments for schizophrenia. Curr Treat Options Psychiatry. 2015;2(3):339-348.
- Dickerson FB, Lehman AF. Evidence-based psychotherapy for schizophrenia: 2011 update. J Nerv Ment Dis. 2011;199(8):520-6.
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-76.
- Morrison AP, Barratt S. What are the components of CBT for psychosis? A Delphi study. Schizophr Bull. 2010;36(1):136-142.
- Foruzandeh N. Occupational therapy for inpatients with chronic schizophrenia: A pilot randomized controlled trial. Nursing Science. 2013;10(1):136-141.
- Wilcock AA. Occupational science: Bridging occupation and health. Canadian Journal of Occupational Therapy. 2005;72(1):5-12.
- Caqueo-Urízar A, Rus-Calafell M, Urzúa A, Escudero J, Gutiérrez‑Maldonado J. The role of family therapy in the management of schizophrenia: challenges and solutions. Neuropsychiatr Dis Treat. 2015;11:145-151.
- Pharoah F, Mari J, Rathbone J, Wong W. Family intervention for schizophrenia. Cochrane Database Syst Rev. 2010;(12):CD000088.
- Fonagy P. The effectiveness of psychodynamic psychotherapies: An update. World Psychiatry. 2015;14(2):137-150.
- Malmberg L, Fenton M. Individual psychodinamic psychotherapy and psychoanalysis for schizophrenia and severe mental illnesses. Cochrane Database Syst Rev. 2001;3:CD001360.
- Temel Y, Hescham SA, Jahanshai A, Janssen MLF, Tan SKHvan Overbeeke JJ, et al. Neuromodulation in psychiatric disorders. Int Rev Neurobiol. 2012;107:283-314.
- Herte F, Züchner M, Weimar I, Gemmar P, Noll B, Bettag M, Decker C. Implantation of electrodes for deep brain stimulation of the subthalamic nucleus in advanced Parkinson’s disease with the aid of intraoperative microrecording under general anesthesia. Neurosurgery. 2006;59(5):E1138.
- Polese D, Fornaro M, Palermo M, De Luca V, de Bartolomeis A. Treatment-resistant to antipsychotics: A resistance to everything? Psychotherapy in treatment-resistant schizophrenia and nonaffective psychosis: A 25-year systematic review and exploratory meta-analysis. Front Psychiatry. 2019;10:210.
- Buchain PC, Vizzotto ADB, Neto JH, Elkis H. Randomized controlled trial of occupational therapy in patients with treatment-resistant schizophrenia. Braz J Psychiatry. 2003;25(1):26-30.
- Todorovic A, Lal S, Dark F, De Monte V, Kisely S, Siskind D. CBTp for people with treatment refractory schizophrenia on clozapine: a systematic review and meta-analysis. J Ment Health. 2020;1-8.
- Morrison AP, Pyle M, Gumley A, Schwannauer M, Turkington D, MacLennan G, et al. Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): an assessor-blinded, randomised controlled trial. Lancet Psychiatry. 2018;5(8):633-643.
- Edwards J, Cocks J, Burnett P, Maud D, Wong L, Yuen HP, et al. Randomized controlled trial of cloapine and CBT for first-episode psychosis with enduring positive symptoms: A pilot study. Schizophr Res Treat. 2011;2011:394896.
- Dellazizzo L, Potvin S, Phraxayavong K, Lalonde P, Dumais A. Avatar therapy for persistent auditory verbal hallucinations in an ultra-resistant schizophrenia patient: A case report. Front Psychiatry. 2018;9:131.
- Grover Sm Hazari N, Kate N. Combined use of clozapine and ECT: a review. Acta Neuropsychiatr. 2015;27(3):131-42.
- Lambrichts S, Vansteelandt K, Crauwels B, Obbels J, Pilato E, Denduayver J, et al.Relapse after abrupt discontinuation of maintenance electroconvulsive therapy during the COVID-19 pandemic. Acta Psychiatr Scand. 2021;144(3):230-237. doi: 10.1111/acps.13334.
- US National Library of Medicine. ECT in ultra-resistant schizophrenia (SURECT). NCT03542903. Retrieved online at https://www.clinicaltrials.gov/ct2/show/NCT03542903. Accessed 23 Sep 2022.
- Moulier V, Krir MW, Dalmont M< SURECT Study Group, Guillin O, Rothärmel M. A prospective multicenter assessor-blinded randomized controlled study to compare the efficacy of short versus long protocols of electroconvulsive therapy as an augmentation strategy to clozapine in patients with ultra-resistant schizophrenia (SURECT-study). Trials. 2021;22(1):284.
- Khouadja S, Ben Soussia R, Younes S, Bouallagui A, Marrag I, Nasr M. Ultra-resistant schizophrenia and potentiation strategies. European Psychiatry. 2017;41S: S772-846.
- Petrides G, Malur C, Braga RJ, Bailine SH, Schooler NR, Malhotra AK, et al. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. Am J Psychiatry. 2015;172(1):52-8.
- Braga RJ, John M, Schooler NR, Bailine SH, Malur C, Mendelowitz A, Petrides G. Continuation electroconvulsive therapy for patients with clozapine-resistant schizophrenia: A pilot study. J ECT. 2019;35(3):156-160.
- Lally J, Breese E, Osman M, Sim CH, Shetty H, Krivoy A, MacCabe JH. Augmentation of clozapine with ECT: a retrospective case analysis. Acta Neuropsychiatr. 2021;33(1):31-36. doi: 10.1017/neu.2020.32.
- Brunelin J, Galvao F, Mondino M. Twice daily low frequency rTMS for treatment-resistant auditory hallucinations. Int J Clin Health Psychol. 2023;23(1):110034.
- Wagner E, Honer WG, Sommer IE, Koops S, Blumberg DM, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation (rTMS) for schizophrenia patients treated with clozapine. World J Biol Psychiatry. 2021;22(1):14-26.
- Siskind D, Honarparvar F, Hasan A, Wagner E, Sinha S, Orr S, Kisely S. rTMS for clozapine refractory schizophrenia- A systematic review and pairwise meta-analysis. Schizophr Res. 2019;211:113-114.
- Wagner E, Wobrock T, Kunze B, Langguth B, Landgrebe M, Eichhammer P,
- et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370-376.
- Corripio I, Roldán A, Sarró S, McKenna PJ, Alonso-Solís A, et al. Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial. EbioMedicine. 2020;51:102568.
- Andrade C. Transcranial direct current stimulation for refractory auditory hallucinations in schizophrenia. J Clin Psychiatry. 2013;74(11):e1054-8.
- Andrade C. Once- to twice-daily, 3-year domiciliary maintenance transcranial direct current stimulation for severe, disabling, clozapine-refractory continuous auditory hallucinations in schizophrenia. J ECT. 2013;29(3):239-42.
- US National Library of Medicine. ECT in ultra-resistant schizophrenia. NCT03542903. Retrieved online at https://clinicaltrials.gov/ct2/show/NCT03542903. Accessed 06 Nov 2022.
- US National Library of Medicine. SMART design to compare antipsychotic treatments in treatment-resistant schizophrenia. NCT04528095. Retrieved online at https://clinicaltrials.gov/ct2/show/NCT04528095. Accessed 06 Nov 2022.
- US National Library of Medicine. Deep brain stimulation in treatment resistant schizophrenia. NCT02361554. Retrieved online at https://clinicaltrials.gov/ct2/show/NCT02361554. Accessed 06 Nov 2022.
- US National Library of Medicine. Combination of NMDA-enhancing and anti-inflammatory treatments for ultra-resistant schizophrenia. NCT05240976. Retrieved online at https://www.clinicaltrials.gov/ct2/show/NCT05240976. Accessed 23 Sep 2022.
- US National Library of Medicine. An adaptive phase II/III, two-part, double-blind, randomized, placebo-controlled, dose-finding, multi-center study of the safety and efficacy of NaBen®, as an add-on therapy with clozapine, for residual symptoms of refractory schizophrenia in adults. NCT03094429. Retrieved online at https://clinicaltrials.gov/ct2/show/NCT03094429. Accessed 06 Nov 2022.
- SyneuRx. Adult schizophrenia trial testing NaBen as an add-on therapy to existing antipsychotics. Retrieved online at https://www.syneurx.com/snd13/. Accessed 06 Nov 2022.
- US National Library of Medicine. Minocycline augmentation of clozapine for treatment resistant schizophrenia. NCT02533232. Retrieved online at https://clinicaltrials.gov/ct2/show/NCT02533232. Accessed 06 Nov 2022.
- Vasiliu O, Marinescu I, Vasile D. Efficacy analysis of the third generation of cognitive-behavioral therapies - a narrative literature review (II). Psihiatru.ro. 2020;63(4):34-39.
- Vasiliu O, Vasile D, Mangalagiu AG, Petrescu BM, Tudor C, Ungureanu D, Candea C. Efficacy and tolerability of calcium channel alpha-2-delta ligands in psychiatric disorders. RJMM. 2017;CXX(2):27-31.
- Numata S, Umehara H, Ohmori T, Hashimoto R. Clozapine pharmacogenetic studies in schizophrenia: Efficacy and agranulocytosis. Front Pharmacol. 2018;9:1049.
Articole din ediţiile anterioare
Contemporary psychotherapy between time and event. A psychoanalytical perspective (I)
The old art of analysis and, subsequently, the interpretation of names – an avatar of etymology (analysis) and hermeneutics (interpretation) – has ...
Therapeutic options in ultra-resistant schizophrenia. Pharmacological interventions (I)
The concept of “ultra-resistant schizophrenia” (URS), or “clozapine-resistant schizophrenia” (CRS), has multiple definitions, and still, there is n...
Contemporary psychotherapy between time and event. A psychoanalytical perspective (II)
There is, of course, a progression in the degree of generality of concepts. As we know so well, the more general a law, the more valuable it will b...
Contemporary psychotherapy between time and event. A psychoanalytical perspective (III)
The theory and practice of psychoanalysis could be circumscribed within the wider frame of the new scientific and cultural paradigms that have mark...