The mechanisms of urinary continence are still controversial and poorly understood. We present in this paper the main concept concerning the physiology of urinary continence. According to the more recent data, we sustain the “Integral Theory of Urinary Continence” of Scandinavians Petros and Ulmsten as the most comprehensive physiology of continence. Based on evidence of the spectacular worldwide spreading of some surgical techniques as “IVS” or “TVT” as the golden standard for all types of urinary incontinence, we are convinced that this fact represents a hope for future.
Anatomia continenţei urinare la femei
The anatomy of urinary continence in women
First published: 15 noiembrie 2015
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.3.3.2015.4833
Abstract
Rezumat
Mecanismele continenţiei urinare sunt încă controversate şi neînţelese pe deplin. În acest articol prezentăm principalul concept care descrie fiziopatologia continenţei urinare. Conform datelor recente din literatura de specialitate, susţinem „Teoria integralistă a continenţei urinare” descrisă de scandinavii Petros şi Ulmstein ca fiind cea mai completă fiziologie a continenţei. Pe baza nivelului mondial de răspândire spectculoasă a unor tehnici chirurgicale ca „IVS” sau „TVT” ca standard de aur pentru toate tipurile de incontinenţă urinară, suntem convinşi că acest fapt reprezintă o speranţă pentru viitor.
Introduction
The mechanism ensuring continence in humans differs from that of primates by its volitional component. Urinary continence in women is ontogenetically unfavored; the erect position, a short urethra, childbirth injuries are conditions that make fragile or inefficient the voluntary control, resulting in a pathology that affects 15-52% from the female population, 3-17% of which developing severe forms of urinary incontinence(1).
The International Society for Continence (1999) defines UI as involuntary loss of urinary fluid within a stabile vesical reservoir, keeps closed due to a sphincterian mechanism and a competent urethral conduct. Any morphologic or functional alterations of these elements may induce involuntary loss of urine.
Urinary incontinence can manifest as:
-
Stress urinary incontinence (SUI)
-
Urinary incontinence due to detrusor instability(urge UI)
-
Mixed urinary incontinence.
The mixed form of incontinence represents 55.5% of all the incontinence cases. Stamey affirms that up to 2/3 of all cases having urinary incontinence with surgical indication present urge phenomena and that they can be treated by bladder neck suspension(2).
WHO considers UI to be a pathological condition and not a sign or a symptom, this vision being generated by its plurifactorial etiology and its pathology linked to pelvic floor dysfunctions. The mechanism of continence is still insufficiently understood, and is a natural tendency to distinctly consider the “anatomical” and, respectively, the “functional” component of the incontinence, as well as the therapeutic approach. This concept orientated the UI therapeutic principles towards a sum of rules that reserved surgical techniques to the cases having an anatomical cause, the rest beneficiating from conservative therapy. As a matter of fact, the two pathogenic types may combine, so that the concept of mixed UI appeared. The modern pathogenic classification consists of two main forms: anatomical UI and mixed UI. The experience of the last 10 years demonstrated that this view was wrong because the “Integrative Pathogenetic Theory” proves that in the mild or severe forms of UI, no matter the clinical manifestation, surgical procedures represent the main therapeutically solution. This theory is based on the fact that UI is the final result of pelvic floor dysfunctions caused by the damages of supporting structures due to deficient collagen synthesis.
Pathogenic concept
The attempts to decipher the pathogenic mechanism have been concentrated on the form of urinary incontinence appeared during physical effort, considering that other forms of incontinence are generated by functional disorders consisting especially in alterations of the nervous mechanisms of control.
Kennedy (1937) thought that involuntary urine loss during efforts is due to the incompetence of the vesical sphincter. In the early 1950s, Jeffecoate and Roberts demonstrated that urinary losses appear consecutive to the posterior opening of the urethro-vesical angle, following anatomic alterations of the bladder base support. In 1960 Lapides observed the phenomenon of “funneling”in women having stress urinary incontinence, which normally is manifest during micturition. In 1961, Enhorning elaborated the theory of “common manometric premises” that would represent the main pathogenic concept unanimously accepted until the beginning of the ‘90s. According to that, urinary continence during effort is ensured as the proximal urethra is located above pelvic diaphragm, hence being submitted throughout physical effort to the same pressional variations as the bladder. The impact of this theory on the therapeutic approach was of a particular importance judging by the fact that the majority of surgical direct or indirect suspension procedures of the bladder neck have been imagined under the influence of this theory.
The proximal urethra concept
The pathogenic theories developed until the beginning of the ’90s structured the so-called “proximal urethra concept”, sustaining the fact that the neuromuscular and hydrodynamic phenomena in this segment of urethra are responsible for the continence maintenance, and the intraabdominal location of urethro-vesical junction guarantees continence during physical stress.
Consecutive research demonstrated that no connection is to be found between urethral hypermobility and urinary incontinence. Lapides’s theory postulating that the opening of the vesical neck could be one of the main causes of SUI was proven incorrect by Versi (1983) who noted that it is a favoring factor and not a determining one. In 1991, Lose demonstrated by means of multichannel urodynamic studies that the urethra does not influence per se the continence maintenance. In 1999, Shafik communicated a sum of results which deny the proximal urethra concept, demonstrating that urethro-vesical junction is anatomically infralevatorian and, consequently to this topography, the urethra is protected against the effect of the intraabdominal pressure which could have a negative impact over continence.
According to these facts, Green established as pathogenically forms of SUI: typel resulted from anatomical alteration of bladder base corresponding to opening of urethro-vesical angle (X-ray imaging) and typel lincontinence due to urethral hypermobility. Later in 1980, McGuire described typell incontinence without any anatomical modifications recorded clinically or by bead-chain urethrocystography. This type of incontinence results as a defective sphincterian mechanism of bladder neck known as “an intrinsic sphyncterian deficiency” (SDI). The actuarial pathogenic classification of SUI recognized two main forms: SUI due to urethral hypermobility and SUI due to sphincter deficiency.
The medial urethra concept
At the beginning of the ’90s the pathogenic concepts have known a major transformation as a result to the new-born integrative theory announced by the Scandinavian authors Petros and Ulmsten(3,4). Accepting this theory was difficult due to the conceptual inertia generated by the therapeutic successes following the surgical procedures sprung from the common manometric premises theory. In 1993 the same two authors reviewed their theory and made public its final form along with the results confirmed by the experimental research conducted on a series of new minimum invasive surgical techniques (TVT, IVS) which conquered today the entire medical world. Though it does not define it specifically, the integrative theory lays the foundation of the newer “medial urethra theory” postulating the leading role of this anatomical segment in continence maintenance. Anatomical and functional arguments corroborate this innovating concept. The main sphincterian elements (the rabdosphinter and the compressor urethrae) are situated at this level, along with the urethral sustaining structures the pubourethral ligaments (DeLancey, 1994)(9), muscular fibers which make a direct connection with the pelvic diaphragm (Huisan, 1989) and the submucous plexus (Asmunsen and Ulmsten, 1993)(4). Urodynamic studies confirm this concept: at the level of the medial urethra, maximal urethra closure pressure values are recorded during effort (Asmunsen and Ulmsten, 1976), or consequently to the voluntary cease of the urinary flow (Westby 1989, Constantinou and Gonvan, 1976). The medial segment of the urethra transverses the pelvic diaphragm and during effort is compressed laterally by the anterior fascicles of pubococcygeal muscles (Benson, 1993).
The dual concept (mid-proximal urethra)
The evidence that recognize the anatomical and functional role of the medial urethra in the assurance of the continence cannot exclude the part played by the sphincterian complex located at the level of the proximal urethra. Recently, Dorschener (2001) has identified the internal sphincter at the vesical neck, M. sphinctervesicae as this author named it, viewed separately to the detrusor fibres which go around the bladder neck and previously considered to be the sphincterian element (DeLancey).The internal bladder sphincter has the role to tighten the proximal urethra between micturitions and throughout physical stress. Urethro-vesical junction mucous membrane is very sensitive in contact with urine, due to the chemoreceptors within it. A single drop of urine came into contact with proximal urethra initiates detrusor contraction of the striated sphincter. This is a normal faze of micturition that can occur pathologically in case of ISD when the sphincter become incompetent. The direct consequence is detrusor instability(5).
In 1996, Hemaly developed a similar concept postulating that SUI is the result of internal sphincter anatomical damage, consisting in ruptures or lacerations of the connective tissue layer within the internal urethra sphincter. If these lesions are focused at the vesical neck, detrusor instability may appear as a result of urine reaching the proximal urethra. This phenomenon may be manifested separately or associated to SUI. The same situation may be encountered after surgical cure if proximal segment are ignored in favor to medical segment. When the lesions of urethral wall are situated in the medial segment, with an obvious dilation at this level, and the proximal one is intact, the SUI is present. When the entire urethral wall is impaired, the consequence is mixed urinary incontinence(6).
Conceptual dualism appears to be a feasible alternative upon which the main pathogenetic SUI forms can be defined:
-
SUI due to medial urethra incompetence (MUI):
- urethral hypermobility
- defects in pressure transmission
· rigid vaginal wall syndrome
· defects in urethral yightening
-
SUI due to proximal urethra incompetence( PUI)
- bladder neck deficient support
- intrinsic sphincteric deficiency.
These pathogenic forms may be encountered in any combination, therapeutic approach being orientated by the predominant element.
The concept of detrusor instability
Detrusor instability is the main functional UI form that can clinically manifest itself as SUI. This concept is defined by the data offered by urodynamic studies on the bladder filling faze demonstrating that the urine losses may appear at variable time - intervals up to 0.25 sec. After physical effort, explained by uninhibited detrusor contraction.
Until 1993, when the integrative theory on continence was formulated, detrusor instability was thought to be the result of neuromuscular damage. Scandinavian authors have demonstrated that the complaints thought to have a functional cause are, in fact, due to anatomical lesion which can benefit from a form of surgical cure. “Detrusor tranquility” during filling faze is obtained by efferent nervous impulses inhibition at medullar, pontine and cortical level, depending on the intensity and quality of the afferent impulses.
The role of pelvic diaphragm
The pelvis diaphragm (PD) provides support and ensures continence of the urinary and inferior digestive tracts. The presence of the genital tract radically modifies the female pelvis (the fibromuscular floor contains a cleft for the birth canal and excretory drainage - the urogenital hiatus). Pelvic organs are maintained in a stable position by their own fibrous, nervous and vascular structures giving them the possibility to function independently.
PD mainly consists in levatorani with its four divisions - muscular fascicles named after their origin on the pelvic rim: iliococcyceal, ischiococcygeal (coccygeal), pubococcygeal and puborectal muscles. These fascicles interlace on the midline forming a “hammock” supporting the uterus, the bladder and the rectum. From anatomical and functional point of view, PD has a supporting component, situated posterior to the rectum, the levator plate, and a contractive one - the pubococcygeal U-shape layer.The muscular fibers that delimitate the urogenital hiatus span the opening between the bones, in an anteroposterior manner, forming the puborectal muscle. The pubococcygeal U-shape layer forms a true common sphincter that laterally compresses the pelvic organs. Shafik (1999) developed the theory of double sphincterian control, according to which the continence of pelvic viscera is the result of the combined simultaneous or successive action of PD and sphincterian individual pelvic complexes.
PD is a complex structure, its muscular, nervous and connective components being interdependent in their function and offering an active/ passive support to the urethrovesical junction, the vagina and the anorectal junction. Throughout effort PD acts as a “pelvic trampoline” (Zacharin 1981), the muscular contraction starting ~0.25 sec before the voluntary effort initiation. The muscular structures of PD are created by rapid response fibers, active during physical stress and high energy consumers, and by slow fibers, necessitate a lower amount of energy and ensuring PD tonicity. As women are growing old, a variable percent of the rapid fibers are transformed into slow fibers, the PD capacity to stand physical effort being progressively reduced.
The main musculo-connective structures of PD put into tension and maintain the spatial location of urethra, vagina and rectum. When the balance between these forces disappears, various dysfunctions of the anatomical segments become manifest: incontinence, over continence or algic complaints. The keystone in maintaining urinary continence is the muscular stretching of a vaginal segment known under the name of “critical elasticity zone”. The urethra has its own supporting element, the pubourethral ligaments, the rest deriving from those of the vagina, especially the superior level elements and the insertion of endopelvic fascia to its tendinous arch(7).
The role of the connective system
The pelvic diaphragm and the supporting elements of the main pelvic organs are anchored to the pelvis walls by connective structures - fasciae and ligaments. They are formed by connective tissue along with smooth muscle fibers which give them a dynamic quality helping the tonic maintenance of pelvic viscera spatial disposition. The composition of the connective tissue has individual characteristics genetically determined and it also responds to endogenous factors, among which the receptivity to estrogens is characteristics to women (a type estrogen receptors ER a) were originate in trigonal urothelium, submucous urethral plexus, and vaginal epithelium and also in the structure of pubourethral, uterosacral and cardinal ligaments, of pubococcygeal muscles.
The postmenopausal estrogenic insufficiency does not seem to affect the collagen metabolism, but appears to play an important role in maintaining a normal sensorial threshold for the entire urinary tract, and in preserving the tonicity of the urethral wall by their effect on the suburethral vascular plexuses. In menopaused women, the most important collagen alteration consists in diminished hydroxiproline excretion and enhanced number of cross-links within the fibrilar component, resulting in reduced elasticity of the ligamental structures(8).
Control levels of urinary continence
Urinary continence at rest and during physical effort is due to mechanisms organized on two levels: the biomechanical control level (neuromuscular, hydrodynamic) and the neuromuscular control level.
In order to be efficient, which means to ensure the continence, the two levels of mechanisms have to meet the following criteria:
-
the intrinsic sphincterian mechanism located between the vesical neck and the urethra - providing the involuntary continence - must be undamaged;
-
intact extrinsic sphincterian mechanism present in the distal and medial urethra, involuntarily contracted as a reflex response to the height abdominal pressure during effort, or voluntary contracted to stop the urinary flow when necessary:
-
structural and functional integrity of levatorani that connects the pelvic floor to the sphincterian urethral and anal mechanism; n anatomical and functional integrity of the innervating structures (derived from the nervous routes having S-S4 spinal origin);
-
a stable bladder with a normal compliance;
-
normal transmission of the intraabdominal pressure to the proximal urethra.
The biomechanical control of urinary
continence
Systems that ensure continence
Urinary continence results from keeping within the vesical reservoir the accumulated fluid; the presence of a competent urethral tube, which prevents urine gravitational leakage at rest and urinary involuntary losses during effort, helps reaching this goal. Two major systems provide mechanical control of urinary continence:
-
Intrinsic system, resisting to the urinary flow, formed by the sphincterian elements of the proximal urethra, the urethral mucous membrane and the submucous vascular plexuses.
-
Extrinsic system, represented by the sphincterian elements of the medial urethra, the striated periurethral fibers (SPMF), the pelvic diaphragm muscles, pubourethral ligaments, the vaginal wall with its supporting elements (uterosacral ligaments and superior paracolpium) and the endopelvic fascia which spatially stabilizes and functionally connects proximal and medial urethra to the anterior vaginal wall. Voluntary control micturitions are made possible by this system.
When these conditions are not yet met, urinary incontinence may appear, with three major complaints:
-
altered urine collecting function, most frequently due to neurogenic or idiopathic detrusor hyperactivity;
-
insufficient urethral sphincterian mechanism;
-
inadequate neural control of collecting and emptying function of the bladder and urethra.
The urethral support
The bladder and the urethra are maintained in normal anatomical position by their connections to the pelvic walls through the fascial structures (the urethropelvic ligament and the vesicopelvic ligament).
The fusion of the periurethral and perivesical fascia with the covering fascia of levatorani creates these structures, their contraction ascending the urethra during effort or descending it throughout micturition. The urethra and the urethrovesical junction have a hammock-like support in the anterior vaginal wall by means of the endopelvic fascia inserted on the arcus tendineus fasciae pelvis (ATFP). The cardinal ligaments and the superior part of the paracolpium contribute to the vaginal support, hence to that of the urethra. The lacerations causes by the obstetric trauma or the vaginal wall laxity may produce proximal or medial urethra hypermobility, and consequently urinary incontinence.
The anterior vaginal wall has an important supporting role, as an element transmitting to the urethra the posterior visceral pressure, and also as a support of the bladder base and neck. Petros and Ulmsten describe a segment in the proximal urethra and the bladder base, “critical elasticity zone”, which plays a capital part in closing the urethra and vesical neck(3,4).
Mechanisms ensuring continence
The main phenomena ensuring urinary continence are:
-
urethral cooptation
-
bladder neck closure
-
voluntary closure of the urethra.
For these three elements are to act efficiently, it is required the contribution of the extrinsic system which gives connection whit the proximal and the medial urethra intrinsic elements.
1. Urethral cooptation
Anatomic and functional characteristic of female urethra have an optimal ontogenetic project in order to maintain continence at rest and during effort. The urethra is an elastic tube provided with a sphincterian system permitting the caliber and lenght modifications necessary to keep urine within the bladder in the accumulating phase, and its evacuation in micturition. In transversal section the urethra has mucosal folds converging towards the center. Among these, on the anterior versant a prominence is present crista uretralis - representing the resistance zone against which the urethra can be pressed by the striated periurethral musculature in order to reduce its caliber. Immediately underneath the urethral mucous membrane a rich vascular plexus is present, ensuring maximal narrowing of the luminal caliber.
Urethral cooptation is a necessary phenomenon that closes the urethra in front of the urinary flow, provided its anatomical length, caliber and function are preserved. The law of fluids running through elastic tubes postulates that the resistance to flow is directly proportional to the length of the pipe and inversely proportional to its diameter. In order to put up an effective resistance in front of the urinary flow, the length of the urethral tube must be optimal and the diameter minimum. During micturition the urethra is getting shorter by the phenomenon of “funneling”, which reduces the functional length; the relaxation of SPMF enlarges rises, the functional length of the urethra being maintained by closing the vesical neck and reflex contraction of pelvic diaphragm, resulting in the maintenance of the urethral optimal length and the reduction of its caliber.
Closure of the urethra is passively obtained due to the superficial tension offered by the mucus present on the surface of the mucosa; an active role is played in addition by the reduction of the lumen under the mechanical effect of the periurethral striated musculature and of the pelvic diaphragm.
The urethral mucus viscosity and quantity depend on the intervention of the bacterial factor, also being under the influence of the estrogen deprivation. Reduction of the mucus quantity leads to the lowering of the superficial tension; less viscous mucus makes the urethral mucous membrane vulnerable to any chemical or bacterial aggression and trauma(9).
The urethral cooptation may be negatively influenced by the deficiency of the anatomical elements composing the two systems of continence insurance:
-
Extrinsic system:
Altered critical elasticity zone in the anterior vaginal wall
Dysfunctional pubococcygeal muscular fascicles
-
Intrinsic system:
Periurethral striated musculature dysfunction
The quality of the connective tissue
Submucosal urethral vascularization.
Altered vaginal wall elasticity and dysfunctional pubococcygealfibres are mainly related to childbirth trauma or previous surgical procedures; the intrinsic system is affected by menopausal estrogenic deprivation or by degenerative modifications appeared in elderly women.
As a biomechanical phenomenon, the urethral cooptation is obtained at rest by the compression of the urethra through the tonic action of SPMF. During effort the urethral tube is compressed against the underlying strengthened anterior vaginal wall, put under tension by the contraction of anterior pubococcygeal fascicles. Defects in urethral cooptation are due to the abnormal relaxation or rigidity of the vaginal wall al the critical elasticity zone level.
2. Closure of the bladder neck
This is an essential phenomenon to keep the fluid within the vesical reservoir. The closure has two biochemical components: an active one represented by the action of the intrinsic proximal urethra sphincter, and a passive one, indirect, ensured by the pelvic diaphragm contraction.
The sphincterian mechanism
The proximal urethra intrinsic sphincter is formed by smooth muscular fibers and it is a distinct anatomical entity, different from those of the detrusor fibers. Its action consists in maintaining closed the urethral segment found immediately under the vesical neck; these smooth fibers have a tonic action, under sympathetic control. The intrinsic sphincter is sufficient to ensure continence at rest.
Mechanical closure of bladder neck
This mechanism was described by Petros and Ulmsten and represents an additional element ensuring continence during effort(3). Bladder neck closure is realized by elongating proximal urethra, in an inferior and posterior direction, by the muscular pelvic diaphragm. Contraction of the anterior pubococcygeal fascicle puts into tension the vaginal wall, pushing it towards the posterior urethra and immobilizing it. The pubovesical ligaments act passively and anchor the urethra. Throughout effort, levator plaque contraction puts tension the supralevatorian vagina.The lateral aspect of pubococcygeal muscles becomes a semi rigid structure which permits the longitudinal anal muscle (LAM) - connected to the pelvic diaphragm through a connector muscular fascicle - to be tractioned and tipped-up similarly to a flap at the level of pubourethral ligaments. Hence, the bladder base is caudally tractioned and the vesical neck is closed (Figure 1).
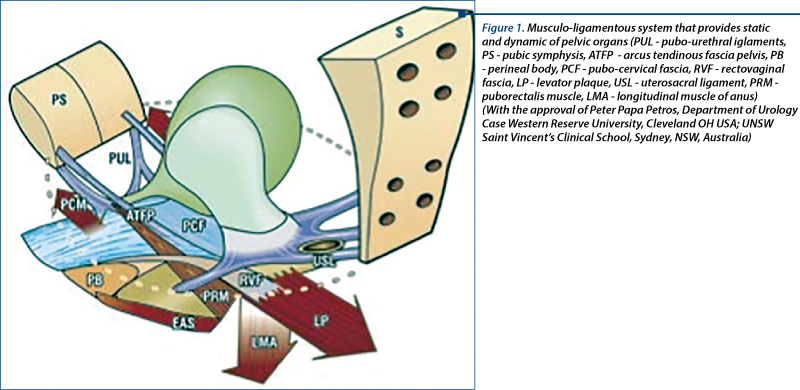
3. Voluntary closure of the urethra
This is a voluntary mechanism realized by the contraction of unspecialized muscles which can be trained by pelvic exercises.
The main muscular component is the puborectal muscle formed by 3 hooks: a superior one, attached to the symphysis pubis (it traction the rectum and the anterior vagina), a medial and attached to the coccyx (which traction the posterior rectum), and an inferior hook, attached anteriorly to the perineal body (it tractions the anterior rectum). The superior hook is the one playing the leading role in urethral closure because its contraction facilitates the action of the second urethral closure mechanism (Figures 2 and 3).
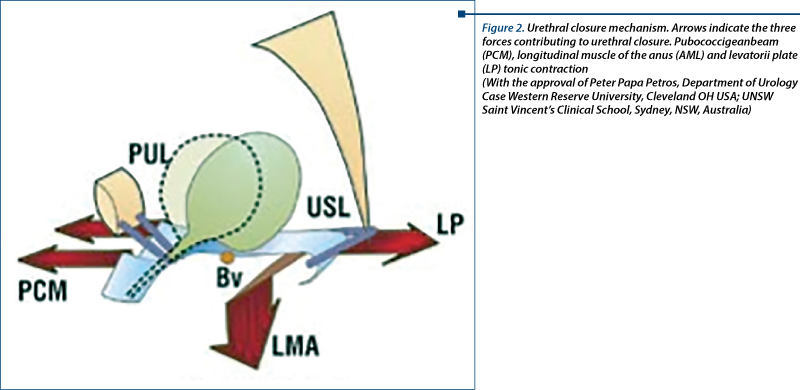
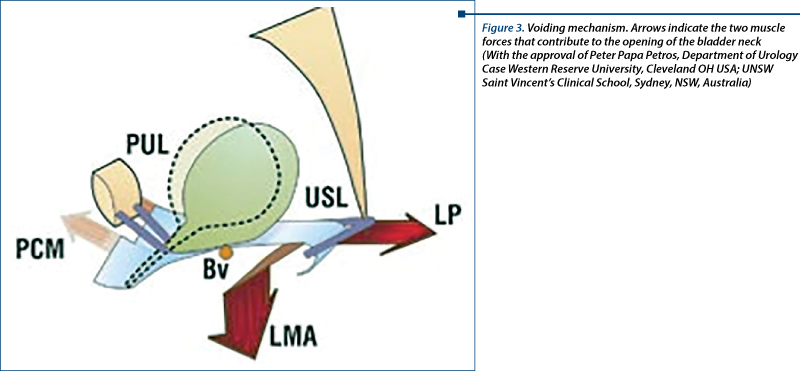
Pressional modification during effort
Physical stress raises the intraabdominal pressure and, consecutively, the intravesical pressure. In order to keep the urine within the bladder, a greater intraurethral pressure is necessary. The excess of urethral pressure, enough to ensure continence at rest, is insufficient during straining. The sphincterian mechanism ensures during effort only 30% of the minimum urethral closure pressure (MUCP), the rest being offered by the indirect transmission of the abdominal pressure to the proximal and medial urethra.
According to the common manometric premises theory (Enhorning), the intraabdominal pressure arises during effort is evenly transmitted to the the abdominalized proximal urethra, hence being ensured urinary continence. Later studies demonstrated that the intraabdominalized position of the proximal urethra is not a necessary condition in order to maintain continence during effort.
Transmission of the abdominal pressure to the proximal and medial urethra is intermediated through the vaginal walls. Among these, the anterior vaginal wall is the one sustaining the urethra like a hammock and it has elasticity variations that can modify the capacity to transmit the posterior visceral pressure. The pressure transmission rates variate between 100% and 120% from the intraabdominal pressure value. Vaginal wall laxity reduces the posterior visceral pressure transmission rate and impedes urethral cooptation and bladder neck closure. Visceral pressure transmission is negatively interfered by the menopausal degenerative modification that leads to naturally developed anterior vaginal wall rigidity(10).
Neuromuscular control of urinary continence
Evolutive stages
The neuromuscular control of the micturitionis defined by the coordination between the bladder filing faze and by their voluntary control. Human being’s mictional mechanism goes through two stages:
-
First stage - during childhood, micturition develops as a reflex spinal act. When the bladder is full, the afferent impulses reaching the spinal cord nervous center start the efferent signal which travels by way of the pelvic nerve (S 2, 3, 4) and stimulates detrusor contraction.
-
Second stage - in adult, as in several domestic animals (dogs, horses), the mother begins to abstain from mictional act. This is possible by getting the habit of maintaining a high a-adrenergic tonus (T10-L2), thus keeping closed the internal urethral sphincter until the time and place are favorable to initiating micturition. When a person wants to urinate, the cortical centers inhibit the high sympathetic a-adrenergic tonus, thus permitting internal sphincter relaxation and bladder emptying by detrusor contraction. If internal sphincter becomes incompetent in front of the effort generated pressure and an involuntary loss of urine appears the record of this event at central level determines a rapid sympathetic response which enhances internal sphincter tonus, stopping an eventual urine loss. The most common cause of weakened internal sphincter is a defective connective tissue generated by ruptures within its fibrilar system. Other causes may be: age-induced atrophy or degenerative alterations due to trauma, infection or hormonal deficiencies. The second stage characterizes the entire life of an individual, the alterations of the micturition mechanisms being the result of a plurifactorial complex acting on a central or a peripheral level. The nervous impulses routes of transmission, the spinal, pontin and cortical nervous centers taking part in storing and eliminating urine, the reflexes developed by their participation will remain stable as long as the information come from the periphery stays correct and these structures anatomically intact(11).
Neuromuscular modulation
The role of nervous receptors
Reception zones for specific stimuli, transmitting afferent impulses to superior control elements, are implied in continence nervous control mechanism. Functional stability of the entire nervous control system depends on the afferent impulses sent from the periphery given the transmission routes are intact.
At the bladder base amongst detrusor fibers, free nervous terminations are heavily concentrated: stretching and pressure receptors (SPR) gathering information about the intravesical pressure and the bladder stretching degree. Nervous amyelinic terminations sensitive to the variations in the chemical composition of the urine and to the presence of inflammation mediators have been identified in the vesical and proximal urethra mucosal membrane. These terminations can initiate afferent impulses which start detrusor contraction when bacterial aggression or Ph variations are present (Morisson 1999). Distal urethra contains tactile and temperature receptor similar to those of the skin.
The zone in the bladder base containing stretching and pressure receptors is anatomically stabile being supported by the anterior vaginal wall corresponding to the critical elasticity zone. During the filling phase, the bladder base remains the less distensible zone, which permits the receptors to stay inactive as long as fluid pressure is not enough to put this zone under tension. Any vaginal wall elasticity alteration will initiate precocious SPR activation and detrusor contraction. This phenomenon explains detrusor instability and functional urinary incontinence.
Conclusions
Urinary continence in women is a complex phenomenon that becomes a voluntarily controlled act over the first two years of life. Mechanical and nervous factors are implied in obtaining continence, their redundancy level having individual specificity. Altered continence mechanism is the result of multiple factors, among which childbirth trauma, menopause and genetic determined connective tissue quality being the most important.
Bibliografie
Stamey TA. Endoscopic suspension of the vesical neck for urinary incontinence in females: report of 203 consecutive patients. Ann.Surg; 1980; 192(4): 465–71.
Petros PE, Ulmsten U. An integral theory of urinary incontinence. Acta Obstet Gynecol Scand Suppl. 1990; 153:7-31.
Falconer C, Ekman-Ordeberg G, Blomgren B, Johansson O, Ulmsten U, Westergren-Thorsson G, et al. Paraurethral connective tissue in stress-incontinent women after menopause. Acta Obstet Gynecol Scand.1998; 77:95–100.
Abrams P, Cardozo L, Fall M. The standardisation of terminology of lower urinary tract functions. Report form the Standardisation Sub-committe of the International Continenece Society. Neurol Urodyn; 2002, 21:167.
Chancellor B. Mapping the future for incontinence treatment worldwide. Rev Urol; 1999, 1(3):145–7.
Fantl JA. Postmenopausal urinary incontinence: comparison between non-estrogen-supplemented and estrogen-supplemented women. Obstet Gynecol; 1988 Jun; 71(6Pt1):823-8.
Glazerner CM, Cooper K. Anterior vaginal repair for urinary incontinence in women. Cochrane Database Syst Rev, 2002;(2):CD003636.
Shafik A. Levator ani muscle: new physioanatomical aspects and role in the micturition mechanism.
World J Urol; 1999, 17(5):266-73.
El Hemaly AKMA. Urethro-raphy: A New Tehnique for Surgical Managementof Stress Urinary Incontinence. http://www.obgyn.net/urogyn/urogyn.asp?page=/urogyn/articles/new-tech-urethro
Mostwin JL, Yang A,Sanders R. Radiology, sonography and magnetic resonance imaging for stress incontinence, Urol Clin North Am; 1995,22:539-49.
Articole din ediţiile anterioare
Eficienţă şi eleganţă versus complicaţii - „banda în H” în tratamentul prolapsului de perete anterior vaginal şi al incontinenţei urinare de efort
Scopul nostru este acela de a raporta rezultatele preliminare ale plasării transvaginale a unei benzi de polipropilenă în 4 braţe la paciente cu pr...