Deep infiltrating endometriosis (DIE) is defined as the infiltration of endometriotic implants deeper than 5 mm from the peritoneal surface. When the lesions infiltrate the muscularis propria layer of the rectum or sigmoid, we talk about intestinal endometriosis (IE), the most common form of extragenital endometriosis, which affects, according to various studies, up to 37% of women with endometriosis. Endometriosis is an underdiagnosed condition mainly due to the lack of cheap and noninvasive diagnostic tools. Although transvaginal ultrasonography (TVUS) is considered the first line in the imaging diagnosis of endometriosis, sonovaginography, endorectal ultrasonography (ERUS), magnetic resonance imaging (MRI) and coloscan are becoming increasingly important in the evaluation of patients with DIE, completing the preoperative assessment of endometriotic lesions, an assessment that needs to be done as accurately as possible, both for the purpose of counseling patients and choosing the optimal therapeutic approach.
Diagnosticul imagistic al endometriozei profund infiltrative
Imaging diagnosis of deep infiltrating endometriosis
First published: 22 martie 2021
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.31.1.2021.4328
Abstract
Rezumat
Endometrioza profund infiltrativă (EPI) reprezintă, prin definiţie, infiltrarea implanturilor endometriozice în profunzime mai mult de 5 mm de la suprafaţa peritoneală. Atunci când leziunile se infiltrează până la nivelul muscularei proprii a rectului sau a sigmoidului, vorbim despre endometrioză intestinală (EI), cea mai frecventă formă de endometrioză extragenitală, care afectează, potrivit diverselor studii, până la 37% dintre femeile cu endometrioză. Endometrioza este o afecţiune subdiagnosticată, în principal din cauza lipsei de instrumente de diagnostic ieftine şi neinvazive. Deşi ecografia transvaginală (ETV) este considerată investigaţia de primă linie în diagnosticul imagistic al endometriozei, sonovaginografia, ecografia endorectală (EER), imagistica prin rezonanţă magnetică (IRM) şi coloscanul devin din ce în ce mai importante în evaluarea pacientelor cu DIE, completând evaluarea preoperatorie a leziunilor endometriozice, evaluare ce trebuie realizată cât mai exact posibil, în scopul consilierii pacientelor şi al alegerii conduitei optime.
Deep infiltrating endometriosis (DIE) – defined as the infiltration of endometriotic implants deeper than 5 mm from the peritoneal surface(1-3) – has the following locations, listed in the order of frequency: uterosacral ligaments, Douglas pouch, rectum, sigmoid, rectovaginal septum, vagina and bladder(2,4,5). When the lesions originate in the rectovaginal septum or in the retrocervical area, they can infiltrate the muscularis propria layer of the rectum and sigmoid, resulting the intestinal endometriosis (IE)(4,6), the most common form of extragenital endometriosis, which affects, according to various studies, between 12% and 37% of women with endometriosis(1,7). IE is usually represented by the involvement of the muscularis propria layer and sometimes even the submucosa, while mucosal involvement is found in less than 5% of cases(1). Up to 95% of IE lesions are located in the rectum and sigmoid colon(1,4), affecting more than one intestinal segment in 39.1% of cases, or as an isolated finding (without other pelvic locations) in 20.6% of patients(1).
Up to 60% of the patients with IE have nonspecific digestive symptoms, chronic, noncyclic, even in the absence of parietal invasion, difficult progression of fecal bolus, constipation and painful defecation, changes in bowel consistency, diarrhea, nausea, rectorrhagia, anorexia and weight loss(6,8,9). In the case of implants located in the posterior vaginal fornix, the symptoms include, in addition to painful defecation, the presence of rectal tenesmus and dyspareunia in fixed point(6).
Endometriosis is an underdiagnosed condition mainly due to the lack of cheap and noninvasive diagnostic tools(10). The delay in diagnosing this pathology is a real issue because, on average, 6-7 years will pass from the onset of symptoms until the diagnosis is established(11).
A problem with endometriosis imaging is the level of experience necessary for the assessor in order for him to acquire acceptable rates of sensitivity and specificity(12). At the same time, it should be noted the high cost of some of these procedures if they were to be used as a screening method(11). The preoperative evaluation of endometriotic lesions as accurately as possible is important both for the purpose of counseling patients and for choosing the optimal therapeutic approach(13).
Transvaginal ultrasonography (TVUS) is considered the first line in the imaging diagnosis of endometriosis(13) and is especially useful for highlighting endometriomas, adenomyosis and bladder endometriosis, but it has a much lower sensitivity in detecting endometriotic lesions of the posterior pelvic compartment, vagina, uterosacral ligaments and rectovaginal septum(14,15).
The advantages of TVUS are well known. It’s easily accessible, it has relatively low costs, it is easily tolerated by patients, it allows the detection of small endometriotic foci, it is not influenced by the intestinal peristalsis and has the ability to detect multiple lesions(15,16). The limitations of this imaging method are, however, many and well documented in the literature(12,17), although when performed by an experienced ultrasonographer, the detection rates are significantly increased, especially in the case of DIE(12). The latest ESHRE (European Society of Human Reproduction and Embryology) guide(18) highlights the role of TVUS performed by highly trained personnel in the diagnosis of endometriosis with locations other than ovarian. Thus, in a study comparing routine TVUS with TVUS performed by an experienced ultrasonographer in the same group of patients, the sensitivity was 25% and 78%, respectively(12). Regarding the diagnostic accuracy of TVUS in the case of IE, sensitivities ranging from 67% to 98% and specificities of 92-100% were found(19).
The presence of the following soft markers: ovaries fixed to the uterine body and iliac vessels and not at the same level, negative “sliding sign”, “kissing ovaries”, obliteration of the Douglas sac at TVUS increases the probability of the presence of DIE and adhesions(13).
In women with chronic pelvic pain suggestive of endometriosis, TVUS has value and should remain the first-line examination method, even though the normal ultrasound appearance cannot rule out endometriosis(2).
Considering the limitations of TVUS, especially regarding the diagnosis of DIE, Dessole et al. described a new imaging technique, namely saline sonovaginography (SVG). This involves combining TVUS with the introduction of saline into the vagina in order to create an acoustic window between the transducer and the structures surrounding the vagina, thus obtaining an improved view of the structures in the posterior pelvic compartment. This technique allows the diagnosis of rectovaginal endometriosis with a higher accuracy than TVUS, reaching a sensitivity of 90.6% and a specificity of 85.7%(20). The main disadvantages of SVG are the need for a second examiner to keep the labia majora tight to prevent the saline from leaking out of the vagina and sometimes the significant discomfort felt by the patient. Replacing the saline solution with ultrasonographic gel has the advantages of eliminating the need for the presence of a second examiner and reducing the patient’s discomfort(13,20).
Reid et al. published in 2014 a prospective study on the use of gel SVG (Figure 1) for the diagnosis of DIE after inserting 20 ml of ultrasound gel into the bottom of the posterior vaginal sac using a syringe. This allows the ultrasound examination of the posterior compartment (the posterior vaginal wall, the posterior face of the uterus and cervix, the uterosacral ligaments, the rectovaginal septum, the anterior rectum and the rectosigmoid) and increases the accuracy of the diagnosis, being easier to perform than the two aforementioned techniques(21).
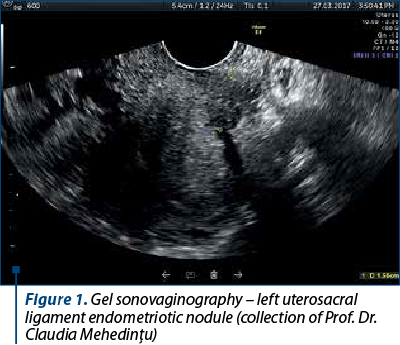
Philip et al. were the first to describe the technique of three-dimensional rectosonography (3D-RSG), a new TVUS technique with intrarectal contrast for the evaluation of IE. When introducing 120 mL of warm water into the rectum, the acoustic window and implicitly the detection rate of endometriotic lesions in the posterior pelvic compartment are increased. Three-dimensional acquisitions can be obtained. After processing, they allow the reconstruction of the endometriotic nodule(22).
Endorectal ultrasound (ERUS), originally proposed in the early 1980s for rectal cancer staging, was first described in 1993 for the evaluation of rectosigmoid endometriosis(4). It can be performed with a rigid probe or a flexible echoendoscope, being the best method of ultrasonographic diagnosis for IE. It allows the evaluation of the extent, depth and height of the intestinal parietal lesion. Using frequencies of 5-7 MHz, a hypoechoic thickening of the muscularis propria can be visualized (the 4th layer from the surface to the depth of the intestinal wall)(1) – Figure 2.
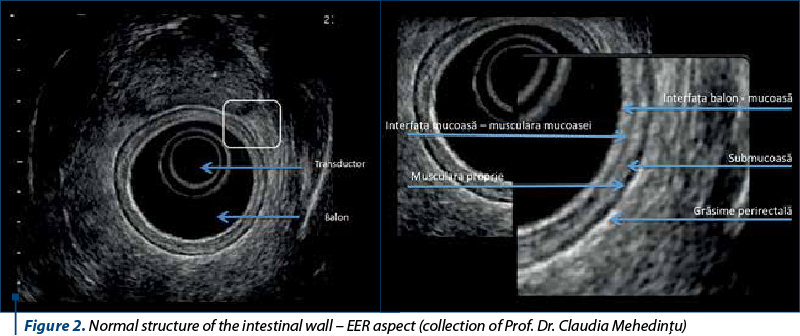
Concomitant colonoscopy and transrectal ultrasound with prior preparation of the colon ± sedation represent a better approach from a technical point of view, as well as of the cost/benefit ratio, allowing the performance of targeted biopsies on a fine needle. The endoscopic examination is performed mainly during the progression with the echoendoscope, and the ultrasonographic evaluation is performed as the probe is slowly withdrawn(4).
The patient is positioned in left lateral decubitus with her thighs and calves bent. After performing a deep rectal examination (to detect the presence of anorectal stenosis and/or nodules located in the anus, rectum, rectovaginal septum, Douglas pouch, cervix and paracervical regions), the transducer is inserted through the anus and immediately oriented towards the posterior. Then the probe is allowed to slide on the sacrum while being inserted 7-10 cm into the rectal lumen. At this point, 40 ml of water are introduced into the balloon connected to the probe. The probe is then gently pushed up (with short up-down movements) towards the distal sigmoid colon. In this position, the left and right iliac vessels and sometimes even the bifurcation of the abdominal aorta can be observed(4).
ERUS performed by an experienced examiner added to the anamnesis, clinical examination and magnetic resonance imaging (MRI) should determine in most cases the degree of rectal and sigmoid involvement, respectively to the level of which layer of the intestinal wall the endometriotic infiltration extends – serosa, muscularis propria, submucosa, muscularis mucosae or mucosa(22). Ultrasonographic measurements should include the depth, width and height of the lesions. The evaluation of the distance in centimeters from the lower edge of the lesion to the puborectal muscle or anal orifice is very important in the proper preoperative evaluation(4).
MRI is becoming increasingly important in the evaluation of patients with endometriosis. It is useful both alone and as a complementary diagnostic method together with TVUS and ERUS, especially in the evaluation of posterior compartment DIE(23) (Figure 3). It is the only investigation that provides information about the entire pelvis and allows the accurate mapping of DIE implants. No consensus was reached on the need for the use of contrast agents, MRI allowing, even without contrast, a fairly accurate preoperative assessment of the extent of the disease, including deep implants and adhesions(24).
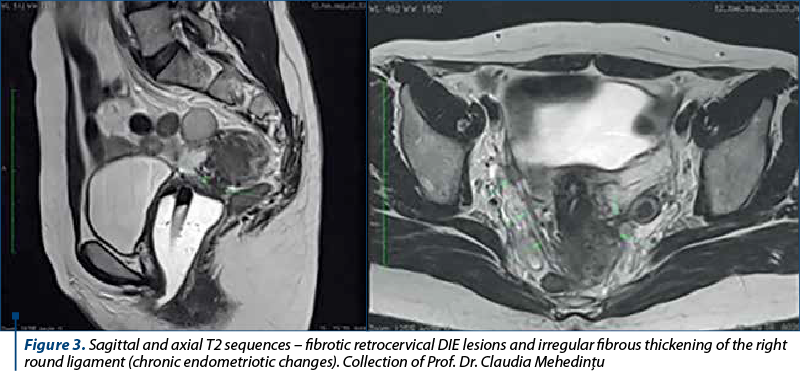
The technique can be augmented by injecting ultrasonographic gel into the vagina (60 ml) and rectum (120 ml) to facilitate the visualization of the dome and recesses of the vagina, rectovaginal septum, and pelvic posterior compartment spaces. High-resolution T2 sequences highlight the fibrotic lesions corresponding to chronic endometriosis and appear as hyposignal T2 (black), while T1 and T1 with fat suppression sequences highlight the endometriotic lesions with high proteinaceous content (hemorrhagic lesions), which appear as hypersignal T1 (white) – Figure 4. These sequences make the differential diagnosis between blood and fat(24).
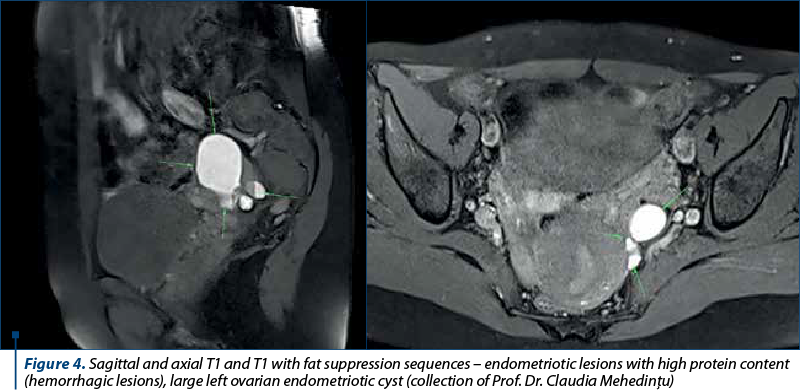
MRI is very useful, but there are some disadvantages, such as the high cost that makes it not easily accessible. Also, it does not allow an interactive, dynamic and in real time evaluation, such as in the case of TVUS or ERUS(23,24).
Computed tomography-assisted virtual colonoscopy/coloscan (CTC) is a noninvasive alternative to conventional optical colonoscopy, allowing a rapid and minimally painful outpatient examination, without the need for analgesia, sedation or contrast administration, that requires prior colon preparation. The entire abdomen and pelvis are scanned, and additional information about extracolonic organs is available (Figure 5)(25).
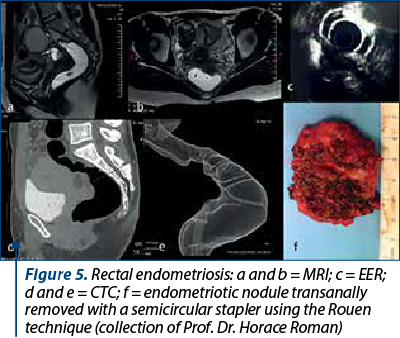
The equipment required to perform the CTC involves a state-of-the-art MDCT (multislice detector computed tomography) scanner coupled with a 3D workstation, on which the virtual colonoscopy software package is loaded, and an automated CO2 insufflation device to slightly dilate the colon during the scan at a pressure of 20-25 mmHg, through a small flexible tube (Foley catheter), inserted in the distal rectum(26).
The patient is scanned in both prone and supine positions in less than a minute to ensure that all segments and surfaces of the intestine are properly visualized, while making the differential diagnosis between the residue and the endometriotic lesion(26).
For those surgeons who choose to preserve the rectum, CTC offers data regarding the rectovaginal septum, intestine and ureter, and facilitates the informed decision-making regarding the surgical technique – conservative versus resection. CTC preoperatively detects multifocal lesions, that may remain undiagnosed laparoscopically, and assesses the degree of stenosis of the digestive tract, which is a major advantage. The information on intestinal stenosis (Figure 5 d, e) suggests the additional risk of occlusive events in women with DIE who want a pregnancy before the surgical attitude(25).
The diagnosis and mapping of DIE lesions require knowledge of pelvic anatomy, and the characteristics of the tissular damage are of paramount importance for the development of the treatment plan. Diagnosing endometriosis remains a challenge, as there is currently no unanimously accepted standard for the diagnostic algorithm. In this regard, it is recommended to combine imaging methods to diagnose and assess as accurate as possible the extent of this enigmatic disease in order to estimate the possible complications and to avoid the unnecessary surgery.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
- Rossini LGB, Ribeiro PAAG, Rodrigues FCM, Filippi SS, Zago R de R, Schneider NC, Klug WA. Transrectal ultrasound – Techniques and outcomes in the management of intestinal endometriosis. Endosc Ultrasound. 2012;1(1):23–35.
- Savelli L, Fabbri F, Zannoni L. Preoperative ultrasound diagnosis of deep endometriosis: importance of the examiner’s expertise and lesion size. Australas J Ultrasound Med. 2012;15(2):55-60.
- Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis and treatment. Fertil Steril. 2012;98(3):564-71.
- Roseau G. Recto-sigmoid endoscopic-ultrasonography in the staging of deep infiltrating endometriosis. World J Gastrointestl Endosc. 2014;6(11):525-33.
- Bazot M, Malzy P, Cortez A, Roseau G, Amouyal P, Darai E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2007;30(7):994–1001.
- Brătilă E, Comandaşu D, Coroleucă C, Cîrstoiu M, Bohîlţea R, Mehedinţu C, Vlădăreanu S, Berceanu C. Gastrointestinal symptoms in endometriosis correlated with the disease stage. ISI Proceedings, XXXVIth National Congress of Gastroenterology, Hepatology and Digestive Endoscopy, Filodiritto Editore. 2016:67-71.
- Berlanda N, Vercellini P, Fedele L. Endometriosis of bowel or rectovaginal space. 2016. www.uptodate.com.
- Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Womens Health. 2015;15(1):39
- Massein A, Petit E, Darchen MA, Lorian J, Oberlen O, Marty O, Sauvanet E, Afriat R, Girard F, Molinie V, Duchatelle V, Zins M. Imaging of intestinal involvement in endometriosis. Diag Intervention Imaging. 2013;94(3):281-91.
- Brüggmann D, Elizabeth-Martinez A, Klingelhöfer D, Quarcoo D, Jaque JM, Groneberg DA. Endometriosis and its global research architecture: an in-depth density-equalizing mapping analysis. BMC Womens Health. 2016;16(1):64.
- Rogers PAW, D’Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis in Montpellier, France. Reprod Sci. 2013;20(5):483-99.
- Fraser MA, Agarwal S, Chen I, Singh SS. Routine vs. expert-guided transvaginal ultrasound in the diagnosis of endometriosis: A retrospective review. Abdom Imaging. 2015;40(3):587-94.
- Guerriero S, Condous G, Van den Bosch T, Valentin L, Leone FP, Van Schoubroeck D, Exacoustos C, Installé, AJ, Martins WP, Abrao MS, Hudelist G, Bazot M, Alcazar JL, Gonçalves MO, Pascual MA, Ajossa S, Savelli L, Dunham R, Reid S, Menakaya U, Bourne T, Ferrero S, Leon M, Bignardi T, Holland T, Jurkovic D, Benacerraf B, Osuga Y, Somigliana E, Timmerman D. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
- Guerriero S, Ajossa S, Minguez JA, Jurado M, Mais V, Melis GB, Alcazar JL. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534-45.
- Piketty M, Chopin N, Dousset B, Millischer-Bellaische AE, Roseau G, Leconte M, Borghese B, Chapron C. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. 2009;24(3):602-7.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal ‘tenderness-guided’ ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452-7.
- Halis G, Mechsner S, D. Ebert A. The diagnosis and treatment of deep infiltrating endometriosis. Deutsches Ärzteblatt International. 2010;107(25):446-56.
- Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12.
- Hudelist G, English J, Thomas AE, Tinelli A, Singer CF, Keckstein J. Diagnostic accuracy oftransvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257–63.
- Dessole S, Farina M, Rubattu G, Cosmi E, Ambrosini G, Nardelli GB. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil Steril. 2003;79:1023-1027.
- Reid S, Lu C, Hardy N, Casikar I, Reid G, Cario G, Chou D, Almashat D, Condous G. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: a multicenter prospective observational study. Ultrasound Obstet Gynecol. 2014;44(6):710-8.
- Philip CA, Bisch C, Coulon A, Pierre de Saint-Hilaire, Rudigoz RC, Dubernard G. Correlation between three-dimensional rectosonography and magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: a preliminary study on the first fifty cases. Eu J Obstet Gynecol Reprod Biol. 2015;187:35-40.
- Roman H, Kouteich K, Gromez A, Hochain P, Resch B, Marpeau L. Endorectal ultrasound accuracy in the diagnosis of rectal endometriosis infiltration depth. Fertil Steril. 2008;90(4):1008–13.
- Bianek-Bodzak A, Szurowska E, Sawicki S, Liro M. The importance and perspective of magnetic resonance imaging in the evaluation of endometriosis. Biomed Res Int. 2013;2013:436589.
- Roman H, Carilho J, Da Costa C, De Vecchi C, Suaud O, Monroc M, Hochain P, Vassilieff M, Savoye-Collet C, Saint-Ghislain M. Computed tomography-based virtual colonoscopy in the assessment of bowel endometriosis: The surgeon’s point of view. Gynecol Obstet Fertil. 2016;44(1):3-10.
- Mehedinţu C, Brînduşe LA, Brătilă E, Monroc M, Lemercier E, Suaud O, Collet-Savoye C, Roman H. Does computed tomography-based virtual colonoscopy improve the accuracy of preoperative assessment based on magnetic resonance imaging in women managed for colorectal endometriosis? J Minim Invasive Gynecol. 2018;25(6):1009-17.
Articole din ediţiile anterioare
Tratamentul medical al endometriozei: este opţiunea medicului?
Introducere. Endometrioza este o patologie cronică benignă, iar managementul ei implică adoptarea unei strategii pe termen lung care include tr...
Calitatea vieţii la pacientele cu endometrioză şi rezecţie colorectală laparoscopică
Obiectiv. Scopul acestei lucrări este de a evalua eficacitatea rezecţiei colorectale laparoscopice în cazurile de endometrioză profundă, precum şi ...
Prezervarea fertilităţii la o pacientă cu polifibromatoză uterină şi endometrioză ovariană. Prezentare de caz
Polifibromatoza uterină este o patologie frecventă, ale cărei diagnostic şi tratament sunt foarte bine documentate, existând numeroase ghiduri elab...
Rolul examinării Doppler în evaluarea patologiei ovariene
olul diagnosticului ecografic în evaluarea tumorilor de ovar este de necontestat. Examenul ecografic are rolul de a evalua caracterele benigne sau ...