Introduction. Endometriosis is a chronic benign pathology and the long-term management plan should include medical treatment and surgical procedures. The current treatment of endometriosis is based on surgery and ovarian suppressive agents. Materials and method. An analysis of the articles published in the specialty literature in order to evaluate the main pharmaceutical options used in the treatment of endometriosis and the recurrence rate of the disease. Results. Oral contraceptives, progestins, gonadotropin-releasing hormone agonists and antagonists, and aromatase inhibitors are the most commonly used agents in the treatment of endometriosis. Hormonal treatments are often associated with unwanted effects, such as delayed conception and recurrence of the disease and symptoms when stopped. The recurrence rate of endometriosis after the surgical treatment is almost 50%. The use of oral contraceptives and progestins was demonstrated to be effective in lowering the recurrence rate of endometriosis. Conclusions. Long-term medical therapy should be recommended for the treatment of endometriosis in order to control symptoms and to prevent recurrence after surgery. After first-line surgery, women should be informed of the high risk of endometriosis recurrence, and they should be invited to seek conception as soon as possible. An alternative is to inhibit ovulation until pregnancy is considered.
Tratamentul medical al endometriozei: este opţiunea medicului?
Medical treatment of endometriosis: is it a choice of the physician?
First published: 13 martie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Gine.27.1.2020.2885
Abstract
Rezumat
Introducere. Endometrioza este o patologie cronică benignă, iar managementul ei implică adoptarea unei strategii pe termen lung care include tratament medical şi terapie chirurgicală. Tratamentul actual al endometriozei se bazează pe intervenţie chirurgicală şi pe terapia de supresie hormonală. Materiale şi metodă. Analiza articolelor publicate în literatura de specialitate pentru a evalua principalele opţiuni farmaceutice utilizate în tratamentul endometriozei şi rata de recurenţă a bolii. Rezultate. Contraceptivele orale, progestativele, agoniştii sau antagoniştii hormonului eliberator al gonadotropinelor şi inhibitorii de aromatază reprezintă opţiunile terapeutice utilizate cel mai frecvent în tratamentul endometriozei. Tratamentul hormonal este asociat frecvent cu efecte nedorite, cum ar fi întârzierea obţinerii unei sarcini sau recurenţa simptomatologiei şi a bolii la oprirea tratamentului. Rata de recurenţă a endometriozei după tratamentul chirurgical este de aproape 50%. Utilizarea contraceptivelor orale şi a progestativelor are o eficienţă demonstrată în scăderea ratei de recurenţă a andometriozei. Concluzii. Terapia medicamentoasă pe termen lung trebuie recomandată în tratamentul endometriozei pentru controlul simptomatologiei şi pentru a preveni recurenţa după intervenţia chirurgicală. După intervenţia chirurgicală iniţială, pacientele trebuie informate în legătură cu riscul crescut de recurenţă a endometriozei şi trebuie îndrumate spre obţinerea unei sarcini cât mai curând posibil. Ca alternativă, tratamentul de inhibiţie hormonală trebuie recomandat până la momentul dorinţei de a obţine o sarcină.
Introduction
Endometriosis is a benign pathology of the female reproductive tract that is characterized by the implantation of endometrial glands and stroma outside the uterine cavity. This pathology is associated with two main manifestations: infertility and chronic pelvic pain of varying intensity. These two aspects affect the quality of life, ranging from mild to severe(1).
The current medical therapies options interfere with estrogen production and, due to that, they cause atrophy to endometriotic implants and stop bleeding. Combined contraceptives, progestins, aromatase inhibitors and gonadotropin-releasing hormone analogues can be used as a therapy option in the treatment of endometriosis. Sadly, they come with side effects, such as recurrence of endometriosis and hypoestrogenic state(2,3).
In patients who haven’t received surgical intervention, the most frequent complaints – such as chronic pelvic pain, dysmenorrhea and dyspareunia – are evaluated using the Visual Analogue Scale (VAS) or Visual Rating Scale (VRS). Another important aspect to consider when treating patients with endometriosis is the evaluation of the quality of life. The most frequent questionnaire used in studies is represented by the SF-36 score.
Oral contraceptives
Oral contraceptive (OC) pills promote atrophy of endometrial lesions through decidualization(4). Oral contraceptives are prescribed in the early stages (minimal and mild) of the disease for pain relief(5). A low dose of oral contraceptivea ameliorates dysmenorrhea and other pain symptoms like non-menstrual pain and deep dyspareunia, as a large randomized study showed(6).
In a multicentric randomized study, 100 patients with endometriosis were assigned to receive oral contraceptives cyclically for three months or placebo treatment(7). The patients were followed-up for three months in order to assess dysmenorrhea using Visual Analogue Scale, repercussions on daily activities and analgesic consumption(7). The results of this study demonstrated that oral contraceptives decreased dysmenorrhea significantly in comparison with the placebo group – VAS: 9 versus 3.1 points, composite pain score: 2 versus 0.7 points(7).
A study published in 2008 on 277 patients evaluated the benefits of long-term oral contraception after surgical intervention for laparoscopic endometriosis cystectomy(8). Out of this cohort, 102 patients received treatment for the entire duration of the follow-up, 129 patients discontinued the treatment, and 46 patients refused the treatment(8). There was a statistical significant difference between patients who received continuous treatment and patients who refused the treatment(8). The recurrence rate for endometriotic cyst was 9 out of 102 patients who received continuous treatment, and 26 out of 46 patients who refused the treatment (56%)(8).
Some authors suggest to substitute the combined contraceptive pills with progesterone-only pills in the postoperative phase, who can better control pain and induce the regression of endometriosis(9).
The administration of oral contraceptives can be cyclic or continuous. The continuous use has some advantages (reduction of dysmenorrhea) compared with the cyclic use. But when it comes to other symptoms or ovarian endometrioma recurrence rate, there are no statistical differences(10). A randomised controlled study demonstrated that long-term administration in a cyclic use of contraceptives had a lower rate of recurrent dysmenorrhea(11). One of the disadvantages of continuous use is that it can lead to metrorrhagia and to prolonged pain(12).
Among most common side effects of the contraceptive pills are headaches, nausea, breast tenderness and mood changes. Regarding the risk of blood clots, the difference between the women who do not take pills and the ones who take the pills is not major (2 women in 10,000 versus 5 to 12 in 10,000)(13). We can say the same thing about the risk of breast cancer (100 in 10,000 versus 110 in 10,000)(13).
Progestins
The intrauterine device (IUD) with levonorgestrel provides the improvement of dysmenorrhea scores in patients with endometriosis by releasing up to 20 µg of active substance. This mechanism promotes, in a first phase, the decidualization of the endometrial implants(14). In a second phase, they induce atrophy(5). Progestins induce the transformation of potent form of estrogens to a weaker form and can promote the inhibition of matrix metalloproteinase. A systematic review on the use of levonorgestrel after the initial surgical treatment provides information regarding the pain scores(15). One of the main benefits of this therapy is that it provides a good therapeutic option for a period of 5 years without the disadvantages of a hypoestrogenic state(16).
There are several studies in patients who were operated for deep infiltrative endometriosis that demonstrate an improvement in the pain control and a reduction of the rectovaginal lesions(17,18). A prospective non-randomized trial published in 2001 provides information regarding the patients with deep infiltrative endometriosis (rectovaginal septum) who were treated using intrauterine device with levonorgestrel(18). In this study, 11 patients presented with moderate or severe pain(18). All of the patients were evaluated using the reported grade of dysmenorrhea and non-cyclical pelvic pain at 12 months after the treatment(18). Five of the patients reported no dyspareunia and six of them reported weak dyspareunia(18).
In a study which analyzed the therapy of the endometriosis patients after conservative laparoscopic surgery, the authors examined 40 patients who received treatment with levonorgestrel intrauterine device or expectant management(2). One year after the beginning of the study, the results were significantly in favour of the treatment with levonorgestrel compared to the expectant management in terms of dysmenorrhea scores(2). In a postoperative state, a continuous cure with progestins can be made for women who want a future pregnancy(9).
Progestins are safe to be used and they can be prescribed when patients can’t or won’t take oral contraceptives. They should be recommended to women with deep lesions, colorectal nodules or with deep dyspareunia(12,19,20).
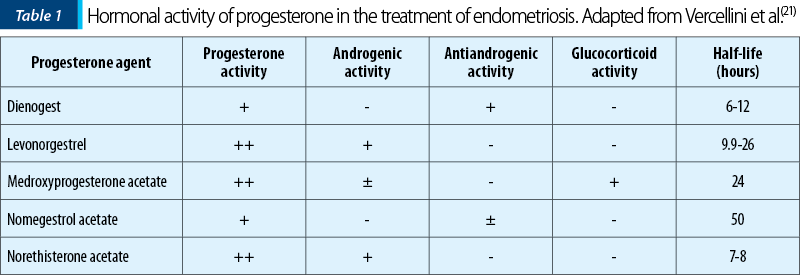
The hormonal activity of different types of progesterone, as well as the half-life time are presented in Table 1(21).
Gonadotropin-releasing hormone agonists and antagonists
The administration of gonadotropin-releasing hormone (GnRH) agonists creates a low estrogen environment by supressing the follicle stimulating hormone (FSH) and luteinizing hormone (LH) production. This mechanism inhibits the proliferation of the endometriotic implants. When GnRH are administered for the treatment of patients with endometriosis, it is recommended to offer an add-back therapy with estrogen in order to prevent the reduction of mineral bone density and to improve the quality of life of the patient. The use of add-back therapy in the context of endometriosis does not reduce the efficacy of the GnRH agonists for the pain symptoms. A study showed that, after 10 years of administration of GnRH agonists with add-back therapy, there was no reduction in mineral bone density(22). Other minor adverse reactions include hot flashes, vaginal atrophy and dryness, headache, vasomotor impairments and an increase in serum lipid levels(23).
The usefulness of GnRH agonists consist in the possibility of administering them in the period before progestogens or occasionally when the patient has pain symptoms or bleeding. They can be also used when there is no response to progestogens and the surgery is not an option(24,25).
GnRH antagonists are effective in the treatment of endometriosis. There are fewer adverse reactions and no estradiol add-back therapy is necessary. The analogues for GnRH antagonists could prove superior to GnRH agonists due to prompt suppression of LH and FSH secretion(26). In a study on 15 women who were administered GnRH antagonists (Cetrorelix®), it was demonstrated that they are useful: all patients had a pain-free period, and regression occurred in 60% of the cases(27).
Aromatase inhibitors
The mechanism of action for aromatase inhibitors addresses the action of aromatase by blocking this enzyme(28). This enzyme converts androgens into estrogens through a physiological process called aromatization(28). In premenopausal women, the main source of estrogen is the ovary, while in postmenopausal women this hormone is produced in the peripheral tissues(28).
The endometriotic tissue has aromatase expression beside the normal endometrium. Aromatase produces estrogen and these high levels of estrogen produced in the pheripheral tissues determine the proliferation and invasion of the endometrial lesions. This promotes pain through a prostaglandin-mediated inflammation(29).
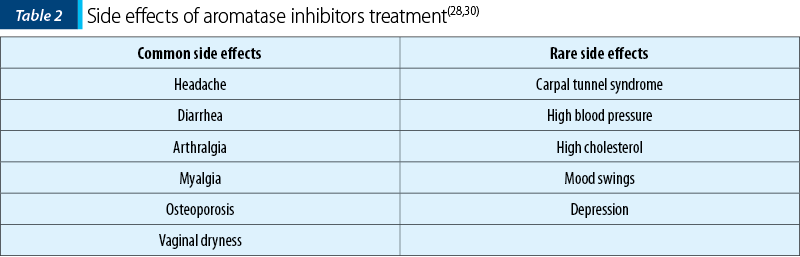
The third-generation aromatase inhibitors, such as anastrozole, letrozole and exemestane, provide a specific effect and reduce the number of side effects associated with this treatment(3). The aromatase inhibitors’ side effects are presented in Table 2(28,30). The administration of aromatase inhibitors in association with another therapy (progestins or OCs) is more efficient than the administration alone, as a systematic review showed(30).
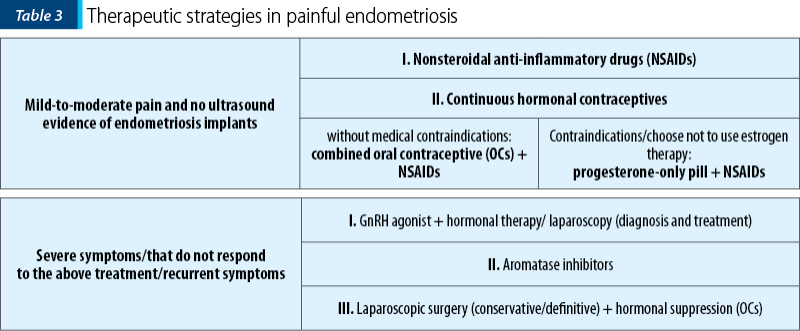
Therapeutic strategies
The medical therapies that are used nowadays have comparable effects as painkillers, but there are differences regarding the side effects, the tolerance and the costs(13). The objective of medical therapy is to alleviate pain. The surgical treatment of endometriosis has an increased improvement on pain symptoms and the quality of life, especially for severe endometriosis. Considering that the disease can reappear, the medical treatment should be used to avoid recurrence of the pain symptoms and endometriosis, but not for improving fertility(31).
The National Institute for Health and Care Excellence (NICE) guideline states that “after laparoscopic excision or ablation of endometriosis, consider hormonal treatment (with, for example, the combined oral contraceptive pill) to prolong the benefits of surgery and manage symptoms”(32).
The first-line medical treatment in painful endometriosis is represented by estroprogestative oral contraceptives and progestins (desogestrel, dienogest, medroxyprogesterone acetate, IUD-levonorgestrel in rectovaginal endometriosis)(33). The second-line treatment options are GnRH agonist with add-back therapy and aromatase inhibitors(33).
Regarding aromatase inhibitors, they should be prescribed only to a limited group of patients who complain of severe pain after surgical procedures and hormonal therapies(31).
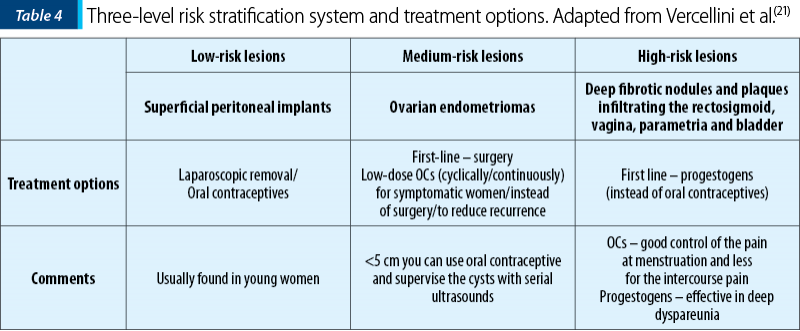
Tables 3 and 4 present two different manners on how to choose the right medical treatment option regarding pain (Table 3) or lesion type (Table 4) as a decisional factor(21).
Rate of recurrence following surgery
and therapeutic options
Even though it is very effective, the surgical treatment of endometriosis is not without risks. Beside morbidity and complications, the recurrence rate of endometriosis after surgery is a threat: after two years the recurrence is around 21.5%, and after five years, 40-50% of patients relapse and they will need another surgery(34,35). Of these patients, 27% will need three or more surgeries that would lead to increased morbidity and would lower the ovarian reserve(36-38). Recurrence prevention is a priority in endometriosis management and is crucial for future fertility. The recurrence rate is different from case to case, and it depends on the severity of the disease and the quality of the surgery(39).
Regarding the hormonal treatment of recurrent endometriosis, NICE guideline states to “offer hormonal treatment (for example, the combined oral contraceptive pill or a progesterone) to women with recurrent endometriosis”(32).
Oral contraceptives and progestins can be used for the prevention of endometriomas, as showed in multiple studies(40,41). For example, in a study on 239 women who have undergone laparoscopic excision of ovarian endometriomas, three groups were formed: continuous users of OCs, cyclic users of OCs, and an observational group(40). The recurrence rate was low in the groups who used oral contraceptives (8.2% in continuous users and 14.7% in cyclic users) compared to non-users (29%)(40). It is a chance that oral contraceptive could be used not only for reducing endometriotic cyst, but also to lower the risk of peritoneal reimplantation due to decreased menstrual flow and endometrial tissue(41). The period of post-surgery administration of oral contraceptives should be long, because the protective effect tends to disappear fast after interruption(8).
The positive effect of the estroprogestins on recurrence of endometriosis is sustained by several studies, and this therapy should be recommended in women who do not want pregnancy(35). This effect is, unfortunately, limited to the period of administration. Thus, the prolonged use of estroprogestins after surgery leads to decreased risk of endometriomas and dysmenorrhea recurrence(35).
In a study that compared the usefulness of dienogest versus goserelin, 198 women were included and followed-up after laparoscopic surgery for endometriosis(42). The study showed that there was a significant difference between dienogest group and the non-treatment group regarding the recurrence rate(42). The side effects were more pronounced in the women with goserelin treatment(42). Thus, dienogest is more effective compared to goserelin in the long-term administration for preventing endometriosis recurrence(42).
Conclusions
Endometriosis is a common estrogen-dependent disorder that can result in substantial morbidity, including pelvic pain, multiple operations and infertility. The current treatment of endometriosis is mainly based on surgery and ovarian suppressive agents, but the management should be individualized according to symptomatology, degree of disease and the reproductive status. The current medical treatments of endometriosis act by blocking ovarian function, suppressing menstruation and inducing endometrial atrophy. Their main limitation is the contraceptive effect for women seeking pregnancy.
The purpose of the medical treatment is to control symptoms, to preserve the residual reproductive potential, to prevent the endometriosis progression, to prevent the new development of endometriosis lesions and progression of adhesions, to avoid serial surgery and, at the same time, it should have a favorable effect on the body and on social and mental well-being.
After first-line surgery, women should be informed about the high risk of endometriosis recurrence and they should be invited to seek conception as soon as possible. An alternative is to inhibit ovulation until pregnancy is considered.
The long-term hormone therapy should be recommended after surgery in order to treat endometriosis and should be used until the woman decides to conceive.
Conflicts of interests: The authors declare no conflict of interests.
Bibliografie
- Allen C, Hopewell S, Prentice A, Allen C. Non steroidal antiinflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2005; (4):CD004753.
- Elnashar A. Emerging treatment of endometriosis. Middle East Fertil Soc Jl. 2015; 20,61–9.
- Tosti C, Biscione A, Morgante G, et al. Hormonal therapy for endometriosis: from molecular research to bedside. Eur J Obstet Gynecol Reprod Biol. 2017; 209:61–6.
- Meresman GF, Vighi S, Buquet RA, Contreras Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl 2, Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000; 74:760–6.
- Pourmatroud E. Medical Treatment in Endometriosis. In: Endometriosis - Basic Concepts and Current Research Trends. 2012; 10.5772/29932.
- Harada T, Kosaka S, Elliesen J, Yasuda M, Ito M, Momoeda M. Ethinylestrdiol 20 μg/drospirenone 3 mg in a flexible extended regiment for the management of endometriosis-associted pelvic pan: a randomized, controlled trial. Fertil Steril. 2017;108:798–805.
- Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008; 90:1583-8.
- Vercellini P, Somigliana E, Daguati R, et al. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008; 198:504e1-5.
- Schindler AE. Hormonal treatment of patients with endometriosis in the postoperative phase. J Pregnancy Reprod. 2018; 2(1):1-2.
- Muzii L, Di Tucci C, Achilli C, et al. Continuous versus cyclic oral contraceptives after laparoscopic excision of ovarian endometriomas: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016; 214 (2):203-11.
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Savelli L, Venturoli S. Long-term oral contraceptive pills and postoperative pain management after laparoscopic excision of ovarian endometrioma: a randomized controlled trial. Fertil Steril. 2010; 94:464–71.
- Vercellini P, Buggio L, Berlanda N, et al. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril. 2016; 106(7):1552–71.e2.
- National Institute for Health and Care Excellence: Hormone Treatment for Endometriosis Symptoms-What Are My Options? Patient Decision Aid. 2017 Sept; Available at: https://www.nice.org.uk/guidance/ng73/resources/patient-decision-aid-hormone-treatment-for-endometriosis-symptoms-what-are-my-options-pdf-4595573197
- Lockhat FB, Emembolu JE, Konje JC. Serum and peritoneal fluid levels of levonorgestrel in women with endometriosis who were treated with an intrauterine contraceptive device containing levonorgestrel. Fertil Steril. 2005; 83:398–404.
- Abou-Setta AM, Al-Inany HG, Farquhar CM. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006; 4:CD005072.
- Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005; 20:1993–8.
- Vercellini P, Aimi G, Panazza S, De Giorgi O, Pesole A, Crosignani PG. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril. 1999; 72:505-8.
- Fedele L, Bianchi S, Zanconato G, Portuese A, Raffaelli R. Use of a levonorgestrel-releasing intrauterine device in the treatment of rectovaginal endometriosis. Fertil Steril. 2001; 75:485–8.
- Morotti M, Remorgida V, Venturini PL, et al. Progestogen-only contraceptive pill compared with combined oral contraceptive in the treatment of pain symptoms caused by endometriosis in patients with migraine without aura. Eur J Obstet Gynecol Reprod Biol. 2014; 179:63–8.
- Vercellini P, Frattaruolo MP, Somigliana E, Jones GL, Consonni D, Alberico D, Fedele L. Surgical versus low-dose progestin treatment for endometriosis-associated severe deep dyspareunia II: effect on sexual functioning, psychological status and health-related quality of life. Hum Reprod. 2013; 28:1221–30.
- Vercellini P, Buggio L, Frattaruolo MP, Borghi A, Dridi D, Somigliana E. Medical treatment of endometriosis-related pain. Best Pract Res Clin Obstet Gynaecol. 2018; 51:68–91.
- Bedaiwy M, Casper R. Treatment with leuprolide acetate and hormonal add-back for up to 10 years in stage IV endometriosis patients with chronic pelvic pain. Fertil Steril. 2006; 86:220–2.
- Dlugi AM, Miller JD, Knittle J. Lupron Study Group. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Fertil Steril. 1990; 54:419–27.
- Kitawaki J, Kusuki I, Yamanaka K, Suganuma I. Maintenance therapy with dienogest following gonadotropin-releasing hormone agonist treatment for endometriosis-associated pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2011; 157(2):212-16.
- Kitawaki J, Ishihara H, Kiyomizu M, Honjo H. Maintenance therapy involving a tapering dose of danazol or mid/low doses of oral contraceptive after gonadotropin-releasing hormone agonist treatment for endometriosis-associated pelvic pain. Fertil Steril. 2008; 89:1831–5.
- Finas D, Hornung D, Diedrich K, et al. Cetrorelix in the treatment of female infertility and endometriosis. Expert Opin Pharmacother. 2006; 7(15):2155–68.
- Kupker W, Felberbaum RE, Krapp M, et al. Use of GnRH antagonists in the treatment of endometriosis. Reprod Biomed Online. 2002; 5:12–6.
- Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006; 85:1307–18.
- Velasco I, Rueda J, Acien P. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol Hum Reprod. 2006; 12:377–81.
- Patwardhan S, Nawathe A, Yates D, et al. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008; 115:818–22.
- Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009; 69:943-52.
- National Institute for Health Care Excellence. Endometriosis: diagnosis and management. 2017 Sept. Available at: www.nice.org.uk/guidance/ng73
- Zito G, Luppi S, Giolo E, Martinelli M, Venturin I, Di Lorenzo G, Ricci G. Medical treatments for endometriosis-associated pelvic pain. Biomed Res Int. 2014; 2014:191967.
- Evers JL, Dunselman GA, Land JA, Bouckaert PX. Is there a solution for recurrent endometriosis? Br J Clin Pract Suppl. 1991; 72:45-53.
- Somigliana E, Vercellini P, Vigano P, et al. Postoperative medical therapy after surgical treatment of endometriosis: From adjuvant therapy to tertiary prevention. J Minim Invasive Gynecol. 2014; 21(3):328-34.
- Cheong Y, Tay P, Luk F, Laparoscopic surgery for endometriosis: how often do we need to re-operate? J Obstet Gynaecol. 2008; 28:82-5.
- Hachisuga T, Kawarabayashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002; 17:432– 5.
- Somigliana E, Ragni G, Infantino M, Benedetti F, Arnoldi M, Crosignani PG. Does laparoscopic removal of nonendometriotic benign ovarian cysts affect ovarian reserve? Acta Obstet Gynecol Scand. 2006; 85:74–7.
- Somigliana E, Busnelli A, Benaglia L, Vigano P, Leonardi M, Paffoni A, et al. Postoperative hormonal therapy after surgical excision of deep endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017; 209:77-80.
- Seracchioli R, Mabrouk M, Frasca C, Manuzzi L, Montanari G, Keramyda A, Venturoli S. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril. 2010; 93(1):52–6.
- Vercellini P, Somigliana E, Vigano P, De Matteis S, Barbara G, Fedele L. Post-operative endometriosis recurrence: a plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online. 2010; 2:259-65.
- Takaesu Y, Nishi H, Kojima J, Sasaki T, Nagamitsu Y, Kato R, et al. Dienogest compared with gonadotropin-releasing hormone agonist after conservative surgery for endometriosis. J Obstet Gynaecol Res. 2016; 42(9):1152–8.
Articole din ediţiile anterioare
Managementul materno-fetal al unui caz cu anomalie anatomică uterină şi sindrom antifosfolipidic obstetrical
Progresul evaluărilor biologice şi ultrasonografice în sarcină are un impact favorabil, demonstrat în cazul pe care îl prezentăm, în ceea ce prive...
Profilul molecular al endometrului eutopic şi ectopic în endometrioză
Endometrioza reprezintă o boală inflamatorie cronică definită prin apariţia ţesutului endometrial în afara cavităţii uterine, care afectează m...
IRM – avantaje şi limite în diagnosticul şi tratamentul endometriozei
Endometrioza este o boală ginecologică multifocală comună, care se manifestă în timpul anilor de reproducere, cauzând adesea dureri pelviene croni...
Utilizarea testelor prenatale neinvazive în evaluarea riscului de aneuploidii fetale în sarcina gemelară cu un embrion oprit în evoluţie în trimestrul I
Testele neinvazive prenatale sunt teste care analizează ADN-ul fetal din sângele matern şi stabilesc riscul de aneuploidii fetale, analizând cromoz...