SARS-CoV-2 – a novel enveloped RNA beta-coronavirus – infects host respiratory epithelial cells. Patients with COVID-19 manifest a spectrum of upper and lower respiratory tract symptoms. Social distancing measures have proven to be effective in reducing the disease transmission. There are limited data on the impact of the current COVID-19 outbreak on women affected during pregnancy and on the fetal impact, but the clinical manifestations of infection in pregnancy seem to be similar to those in non-pregnant adults, with no evidence of higher mortality. To date, no evidence sustains the vertical transmission of SARS-CoV-2 from amniotic fluid, cord blood, placenta and neonatal nasopharyngeal swab samples. For low-risk pregnancy, during current pandemic, antenatal appointments should be reduced to minimum; pregnancy-associated pathology should not be neglected. Non-face-to-face medical counseling should be maximized, thus protecting both the patient and the health care providers. Routine confirmation of cases with SARS-CoV-2 infection is based on the detection of unique sequences of virus RNA by real-time reverse-transcription polymerase chain reaction (rRT-PCR). The management should be symptomatic and supportive, including fluid and electrolyte balance, oxygen supply, antibacterial and antiviral treatment in selected cases, LMWH to all pregnant COVID-19 patients requiring admission, fetal monitoring and individualized timing and mode of delivery. Several vaccines are under development and will be available in the future.
Managementul infecţiei cu SARS-CoV-2 în cazul pacientelor gravide. Cunoştinţele actuale privind COVID-19 în sarcină
SARS-CoV-2 infection management in pregnant patients. All we know by now about COVID-19 in pregnancy
First published: 10 aprilie 2020
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OBSGIN.68.2.3856
Abstract
Rezumat
SARS-CoV-2 – un nou tip de betacoronavirus ARN – infectează celulele epiteliale de la nivelul căilor respiratorii. Pacienţii cu COVID-19 manifestă un spectru de simptome aparţinând tractului respirator superior şi inferior. Măsurile de distanţare socială s-au dovedit a fi eficiente în reducerea transmiterii bolii. Deşi până în prezent există date limitate privind impactul infecţiei cu SARS-CoV-2 asupra femeilor însărcinate şi asupra fătului, manifestările clinice par să nu difere de cele ale adultului în afara sarcinii, nefiind raportată o rată crescută a mortalităţii materne. Pentru sarcinile cu risc scăzut, fără comorbidităţi, în timpul pandemiei de COVID-19 vizitele prenatale trebuie reduse la minimum, fără a neglija însă patologia asociată sarcinii cu risc. Consilierea medicală la distanţă ar trebui maximizată. Confirmarea cazurilor de infecţie cu SARS-CoV-2 se bazează pe detectarea secvenţelor unice de virus ARN prin tehnici de rRT-PCR. Managementul este simptomatic şi suportiv, incluzând reechilibrare hidroelectrolitică, oxigen, terapie antibiotică şi antivirală în anumite cazuri, heparină cu greutate moleculară mică administrată tuturor gravidelor infectate şi internate, monitorizare fetală şi modalitate individualizată de naştere. Mai multe vaccinuri sunt în curs de dezvoltare şi vor fi disponibile în viitor.
Introduction
The new type of coronavirus, SARS-CoV-2, belongs to the family of coronaviruses, positive-stranded RNA viruses, subfamily of b-coronaviruses, and shares 79.5% of the genetic sequence with SARS-CoV, which is the agent of the epidemic that started in 2002(1). So far, there have been identified several types of coronaviruses, which may be the cause of common cold, as well as severe lower respiratory tract infections. The infection is transmitted via direct contact, fomites or aerosol routes. The reported incubation period is between 0 and 24 days(2), but the medium incubation period remains short, at 3 days(3).
In 1918, the influenza pandemic caused a mortality rate of 2-6% in the overall population, but 37% among pregnant women(4). In 2009, pregnant women were responsible for 1% of the patients infected with influenza A subtype H1N1 virus, but they accounted for 5% of all H1N1-related deaths(5). Furthermore, SARS-CoV and MERS-CoV are both known to be responsible for severe complications during pregnancy, including the need for endotracheal intubation, admission to an intensive care unit (ICU), renal failure and death(6). Wong and colleagues also reported that around 50% of pregnant women who developed SARS were admitted to the intensive care unit, approximately 33% of pregnant women with SARS required mechanical ventilation, and the mortality rate was as high as 25% for these women(7).
Due to the physiological changes in their immune and cardiopulmonary systems, pregnant women should be more likely to develop severe illness after an infection with respiratory viruses, but this is not the case for SARS-CoV-2. The clinical characteristics of COVID-19 pneumonia in pregnant patients are similar to those in the general population, divided into mild, normal and severe degrees(8), while, at the same time, asymptomatic infection with SARS-CoV-2 also exists during the outbreak period. No higher risk for pregnant women of contracting the virus, milder infection and good recovery have been reported by now.
Changes of acute lung injury due to SARS-CoV-2 receipted by angiotensin converting enzyme 2 in pulmonary epithelium and endothelium consist in hyaline membrane formation and immune cells (majorly macrophages) infiltrate in alveoli space, focal hemorrhage and interstitial fibrosis in lung tissue. Most of the infiltrated lymphocytes are CD4-positive T cells. Cytokines produced by T-helper (Th) lymphocytes regulate immunity and inflammation. While Th1-type cytokines are microbicidal and proinflammatory (interferon, interleukin IL-1a, IL-1b, IL-6 and IL-12), Th2-type cytokines are anti-inflammatory and comprise in IL-4, IL-10, IL-13 and transforming growth factor beta (TGF-b)(9). In pregnancy, cell-mediated Th1 immunity shifts towards Th2 type, thus predisposing the pregnant patient to be more susceptible to viral infections, but at the same time Th2 response favors the expression of anti-inflammatory cytokines, resulting in a mild form of COVID-19(10). High levels of proinflammatory cytokines, including IL-2, IL-6, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1a and TNF-a, were detected in the patients’ serum(1). The cytokine storm, macrophage and endothelial activation lead to a profound inflammatory state, a prothrombotic state due to effects of IL-1, IL-6 and to immunothrombosis, a new term that defines the existence of hyaline thrombus in small vessels of different organs, with no local evidence of coronavirus.
While there are already numerous cases of SARS-CoV-2 infection worldwide in the general populations, the infection during pregnancy is still an ongoing process of assembling information. We are currently unaware of what impact it may have on early or late stages of pregnancy, and how it may affect the fetus(11). So far, no maternal deaths have been reported, and whether SARS-CoV-2 infection increases the risk of miscarriage and stillbirth is still unknown.
There is currently no evidence of vertical transmission in pregnant women, as amniotic fluid, cord blood samples, placenta and neonatal throat-swab samples collected from the patients have not tested positive for SARS-CoV-2(12). Recent data suggesting vertical transmission as witnessed by IgM antibodies in neonates require confirmation from further research.
In a study first published on May 5, 2020, by a research team from Yale School of Medicine, in the case of a second-trimester pregnancy with symptomatic SARS-CoV-2 infection complicated by severe preeclampsia and placental abruption, after the decision of pregnancy termination, the placenta was analyzed for the presence of SARS-CoV-2 through molecular and immunohistochemical assays and by electron microscopy. SARS-CoV-2 was found localized predominantly in the syncytiotrophoblast cells at the maternal-fetal interface of the placenta. The fetal heart and lungs were also analyzed, but showed no sign of infection. This is the first case of placental infection, highlighting the potential for severe pregnancy complication(13).
In Romania, comparing to other countries, the number of infected patients is low and the number of infected pregnant women is even lower. We have 44 sanitary units designated to admit pregnant patients with effective isolation and protective measures, ready to receive and hospitalize symptomatic suspected and/or probable cases. Currently, we rely on real-time polymerase chain reaction (RT-PCR) for the detection of virus nucleic acid. During the last two months of emergency state of the country, a “Methodology regarding birth control of SARS-CoV-2/COVID-19 suspicion/confirmed infection of pregnant women and medical care of the newborn” has been elaborated by the Health Ministry and applied rigorously in all departments of obstetrics and gynecology(14).
Treating symptomatic patients with suspected or confirmed SARS-CoV-2 infection needs full personal protective equipment (PPE). In our university multidisciplinary emergency hospital, the patients arrived at the emergency room must be sorted based on symptoms and local case definitions, into low or high risk of SARS-CoV-2 infection groups, in order to determine the disposition of the patient and the type of infection control precautions required for the health care providers. The safety of health care providers is utterly important, as it may be the main transmission vector, in the absence of protective equipment.
In our clinic, in the sorting area, all health care providers are equipped with respiratory surgical masks and gloves, while patient history must be done at a safe distance of 1 meter. All attending medical staff is protected by respirator masks, protective eyewear, face protective shield and surgical gown and gloves when providing care for confirmed cases of COVID-19.
Based on the emerging literature, we attempt to provide in this review a simple list of clinical features and laboratory tests, recommended for pregnant women with SARS-CoV-2, thus potentially assisting in the prognostic monitoring of such patients(15-18).
Diagnosis
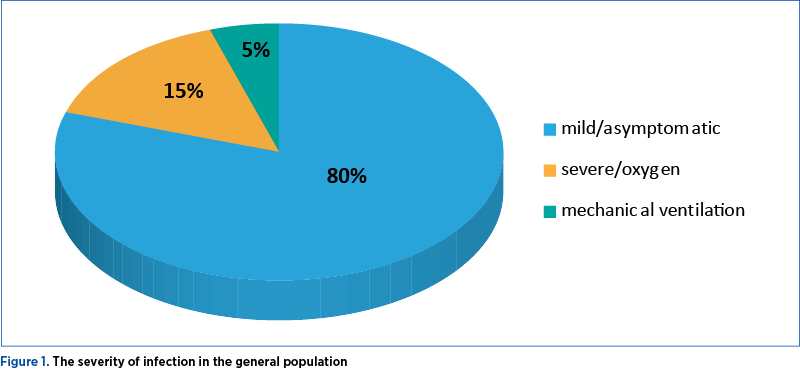
Approximately 80% of infections with SARS-CoV-2 are mild or asymptomatic, 15% are severe, requiring supplemental oxygen, and 5% are critical, requiring mechanical ventilation (Figure 1)(19).
Clinical features
Fever, cough, myalgia, sore throat, malaise and ophthalmic manifestations(20), such as symptoms of conjunctivitis, including eye redness, ocular irritation, foreign body sensation, tearing, and chemosis, are the symptoms which more commonly affected patients with severe systemic symptoms of COVID-19, though they can rarely present as an initial manifestation of the disease. There have been no reports of COVID-19 patients experiencing blurred vision, subconjunctival hemorrhage, eyelid ecchymoses, conjunctival scarring, keratitis or pseudomembrane formation. Dyspnea, diarrhea, pharyngalgia, headache and abdominal pain complete the clinical manifestations.
As of April 27th, 2020, The Centers for Diseases Control and Prevention (CDC) has added three symptoms to a total of nine, from the newly added the most important ones being chills, repeated shaking with chills, new loss of taste or smell. Also, CDC has issued a list of emergency warning signs for COVID 19, in which cases patients should seek immediate medical attention:
-
trouble breathing
-
persistent pain or pressure in the chest
-
new confusion or inability to arouse
-
bluish lips or face(21).
Nucleic acid amplification tests (NAAT) for SARS-CoV-2
The current gold standard for the etiological diagnosis of SARS-CoV-2 infection is real-time reverse-transcription polymerase chain reaction (rRT-PCR) analysis of respiratory tract specimens, but the test has a high rate of false-negative results due to both nasopharyngeal swab sampling error, which often requires repeated sampling, and viral burden. Routine confirmation of COVID-19 cases is based on the detection of unique sequences of virus RNA by NAAT, with confirmation by nucleic acid sequencing when necessary(22). Samples collected from the lower respiratory tract are sputum, aspirate or lavage, and from the upper respiratory tract, naso-/oropharyngeal swabs or nasopharyngeal wash/aspirate. Stools, whole blood, urine and, if deceased, material from autopsy could be considered. Further research is needed to determine the effectiveness and reliability of repeated sampling. The WHO recommendation is that, when possible, lower respiratory tract specimens, such as sputum, endotracheal aspirate or broncho-alveolar lavage, should be collected for SARS-CoV-2 testing, as they have a higher diagnostic value when compared to upper respiratory tract probes of combined nasopharyngeal and oropharyngeal swabs.
Low sensitivity of nasopharyngeal swabs, exposure risks to health care workers and global shortages of swabs and PPE, however, were the main reason for further studies into new diagnosis methods. A new study concluded that saliva is a promising candidate for SARS-CoV-2 diagnostics, as collection is minimally invasive, being easily done at home by the patient, showing also comparable sensitivity to nasopharyngeal swabs in the detection of other respiratory pathogens, including endemic human coronaviruses, in previous studies(23).
In addition to providing confirmation for the presence of the virus, regular sequencing of a percentage of specimens from clinical cases can be useful to monitor for viral genome mutations that might affect the performance of medical countermeasures, including diagnostic tests. Virus isolation is not recommended as a routine diagnostic procedure.
Serological testing
Specific data on the production of IgG and IgM is crucial in order to allow the rapid identification of the infection. Studing past coronavirus infections – as, for example, after SARS-CoV infection –, IgM could be detected in patients’ blood after 3-6 days, while IgG could be detected after 8 days(24). So far, in reported cases of SARS-CoV-2 infection, there seems to be a virus specific IgM peak 7-9 days after the onset of the disease and, by day 14, the presence of IgG antibodies, whilst IgM antibodies tend to disappear(1,25,26). Given the situation, it is of great significance the fact that false negative tests could occur if immunoglobulin values are not high enough at the time of the detection, so we must be careful as 13.5% of unselected attendees are asymptomatic patients that spread the disease. IgG persists well above the cutoff for immunity at 240 days following infection.
Serological surveys can aid investigation of an ongoing outbreak and, in cases where NAAT assays are negative and there is a strong epidemiological link to SARS-CoV-2 infection, paired serum samples (in the acute and convalescent phase) could support diagnosis once validated serology tests are available. Serum samples can be stored for these purposes. Cross-reactivity to other coronaviruses can be challenging(27). Serum for serological testing should be done, once validated and available. Paired samples are necessary for confirmation with the initial sample collected in the first week of illness and the second ideally collected 2-4 weeks later.
The early promise of lateral flow immunoassay (LFIA) devices has been up for questions once concerns about sensitivity and specificity appeared. A study testing plasma for SARS-CoV-2 IgM and IgG antibodies by ELISA versus nine different LFIA devices resulted in the detection of SARS-CoV-2 IgM or IgG in 34/40 individuals with an RT-PCR-confirmed diagnosis of SARS-CoV-2 infection (sensitivity 85%; 95% CI; 70-94%), versus 0/50 pre-pandemic controls (specificity 100%; 95% CI; 93-100%) when using the ELISA tehnique. IgG levels detection presented a sensitivity of 100% with 31/31 RT-PCR-positive individuals tested ≥10 days after the first symptoms. The main conclusion of the study is that the performance of existing LFIA devices is inadequate, whereas ELISA can be calibrated to be specific for detecting and quantifying SARS-CoV-2 IgM and IgG, and is highly sensitive for IgG from 10 days after the first symptoms(28).
Imagistic diagnosis
Imaging modalities play an essential role in the management of suspected COVID-19 patients. It seems that before positive results of rRT-PCR, 60-93% of patients have positive CT findings consistent with SARS-CoV-2 infection. Chest imaging may aid but not replace the molecular confirmation of COVID-19. The predominant findings are peripheral airspace shadowing on a plain chest radiography and bilateral, multilobar ground-glass opacities or consolidation on computed tomography (CT) scan of the chest(10). These features are nonspecific and appear to be similar in pregnancy. A study focusing on the cases of 55 pregnant women who had been initially diagnosed with suspected infection with SARS-CoV-2, out of which 13 were confirmed, revealed that imaging of pulmonary CT scan showed ground-glass opacity in 46.2% of cases, patch-like shadows in 38.5% of cases, fiber shadow in 23.1% of cases, pleural effusion in 5 of 13 cases, and pleural thickening. In a pregnant woman with suspected COVID-19, a chest CT scan may be considered as a primary tool for the detection of SARS-CoV-2 in epidemic areas(29,30).
Radiation-free, point-of-care diagnostic tool, lung ultrasound examination is particularly useful for assessing the lungs of pregnant women. Air from the normal lungs determines the reflection of ultrasound beam that forms parallel, horizontal artifacts, A-lines; the only visible structure, pleura is seen as a hyperechoic horizontal line.
Lung ultrasound is easy, low cost, can be performed at bedside, using portable devices, at the same time and by the same practitioner of obstetric ultrasonography, repeatable over time for longitudinal assessment. The standard approach consists in 14 areas to be examined (6 posterior, 4 lateral, 4 anterior). Lung ultrasound imaging findings in COVID-19 are consistent with viral pneumonia, with diffuse, thick vertical artifacts, B-lines, often bilaterally present. As an inflammatory lung disease, even the early stages of viral pneumonia with SARS-CoV-2 determines an aspect of pleural line usually irregular (blurred), thickened, with a characteristic distribution (monofocal or multifocal, patchy or inhomogeneous involvement, surrounded by spared areas and with no gravitational distribution)(31). Increased density parenchyma characteristic for acute respiratory distress syndrome (ARDS) stage appear as “ultrasonographic white lung”. The consolidation pneumonia pattern of almost completely collapsed lung reveals a solid organ ultrasound aspect, often with variable dimensions of irregular hypoechoic areas. Pleural effusion appears as simple and uniformly anechoic band, or with nonhomogeneous aspect due to the presence of hyperechoic spots determined by blood or fibrin, with or without septa. Pulmonary computed tomographic angiography rule out the thromboembolism(32).
Laboratory findings
Minimum Testing Panel in pregnant women with COVID-19 is presented in Table 1. Currently, there are recommended complete blood count that often reveals lymphopenia, neutrophilia and thrombocytopenia(29), routine coagulation tests, a series of biochemestry and other tests including electrolytes, markers for inflammation, liver, renal and cardiac disfunction. The levels of proinflammatory cytokines, including IL-2, IL-6, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1a and TNFa(1), prolonged thrombin time, hypersensitive troponin I, and serum creatine suggest aberrant coagulation pathway, hepatic, myocardial and kidney injury. The total number of T cells, CD4+ T cells, CD8+ T cells, total B cells, as well as natural killer cells were dramatically decreased in seriously affected patients, suggesting that the severity of lymphocytopenia reflects the severity of COVID-19.
Prognostic factors
While most people with COVID-19 develop only mild or uncomplicated illness, approximately 14% develop a severe disease that requires hospitalization and oxygen support, and 5% require admission to an intensive care unit(7). In severe cases, COVID-19 can be complicated by the acute respiratory distress syndrome, sepsis and septic shock, multiorgan failure, including acute kidney injury and cardiac injury(34). Older age and comorbid disease have been reported as risk factors for death, and recent multivariable analysis confirmed that older age, higher Sequential Organ Failure Assessment (SOFA) score and D-dimer >1 µg/L on admission could help clinicians to identify patients with poor prognosis at an early stage.
The criteria for discharge were the absence of fever for at least three days, substantial improvement in both lungs at the chest CT, clinical remission of respiratory symptoms, and two throat-swab samples negative for SARS-CoV-2 RNA obtained at least 24 hours apart.
The clinical severity of infectious diseases is typically measured in terms of infection fatality risk (IFR), symptomatic case fatality risk (sCFR), and hospitalization fatality risk (HFR). There is a clear and considerable age dependency in symptomatic infection (susceptibility) and outcome (fatality) risks, by multiple folds in each case.
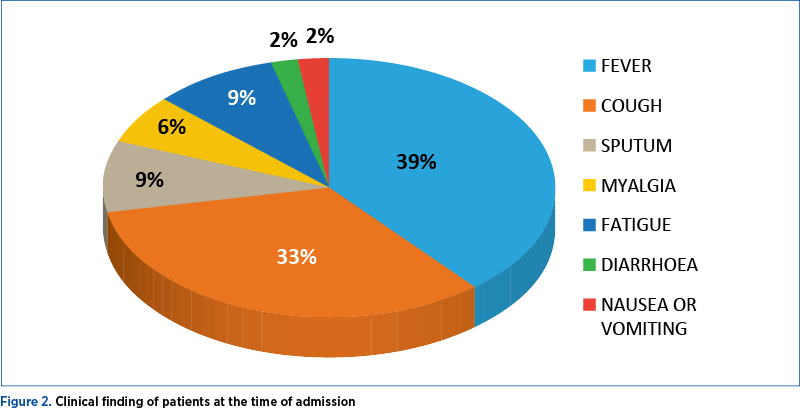
For 656 patients analyzed in three studies, fever (88.7%; 95% CI; 84.5-92.9%), cough (57.6%; 95% CI; 40.8-74.4%) and dyspnea (45.6%; 95% CI; 10.9-80.4%) were the most prevalent manifestations. Among the patients, 20.3% (95% CI; 10-30.6%) required intensive care unit, 32.8% presented with acute respiratory distress syndrome (95% CI; 13.7-51.8), 6.2% (95% CI; 3.1-9.3) with shock, and 13.9% (95% CI; 6.2-21.5%) of hospitalized patients had fatal outcomes (case fatality rate; CFR)(35) – Figure 2.
Treatment
Evan though there is no evidence that pregnant women are more susceptible to SARS-CoV-2 infection or that they are prone to develop severe pneumonia(11), close surveillance is needed, both for the mother and the fetus.
In suspected cases, the general treatment should be insured, such as fluid and electrolyte balance, symptomatic treatment (antipyrexic) and antidiarrheal medicines when required. Vital signs and oxygen levels should be measured, and minimum a test panel for hematology must be done. Fetal monitoring will include a cardiotocography (CTG), as well as an ultrasound assessment for fetal growth and amniotic fluid volume.
In confirmed cases, the management should be symptomatic and supportive, including fluid and electrolyte balance, oxygen supply, antibacterial and antiviral treatment in selected cases, LMWH in all pregnant COVID-19 patients requiring admission, fetal monitoring and individualized timing and mode of delivery; data from Lombardy show that only 10% of deliveries are performed by caesarean section due to severe maternal respiratory insufficiency.
Besides the general principles such as oxygen therapy in order to maintain placental perfusion and prevent fetal hypoxemia(36) (target O2 saturations above 95% and/or pO2 70 mm Hg), administration of intravenous fluid or early mechanical ventilation if needed, the target treatment is desirable, but not existing for the moment. The antiviral treatment seems to be the first option in treating COVID-19. It has been used in China and in other European countries, and it may be used in pregnant patients. Lopinavir-ritonavir has been the preferred drug combination, as it is known to be relatively safe in pregnancy, as revealed by a study of 383 pregnant HIV-positive patients, which found no increase for the risk of fetal anomalies, preterm birth and low-birth weight infants(37). The recommended dose is two capsules (200 mg/50 mg per capsule) twice a day(38). Remdesivir, a nucleotide analog, is currently in phase 3 trials in the United States and China, and so far it seems to be safe during pregnancy(39). Several reports sustain antiviral therapy administration after delivery, due to fetal growth restriction risk(40). Ribavirin is a teratogenic drug that may induce miscarriage, craniofacial and limb defects, and should be avoided, especially in early pregnancy(41). Baricitinib, a Janus kinase inhibitor, has been considered as a potential drug for the treatment of COVID-19. However, it seems to have embryotoxic efects(42). Also, the treatment with Arbidol® was initiated, but with unclear results(43). Chloroquine, the antimalarial drug, seems to be speeding up the recovery, both from a clinical and radiological point of view. Although it crosses the placenta, it may be safely used in all trimesters of pregnancy, with no increased risk of adverse perinatal outcomes(41). In combination with azithromycin and oseltamivir, it may be a promising option(44). The RECOVERY trial of the Oxford University is testing the following drugs against COVID-19: lopinavir-ritonavir, low-dose dexamethasone, hydroxychloroquine, azithromicin and tocilizumab, an anti-IL-6 monoclonal.
At the same time, antibacterial treatment may be needed because of the risk of superimposed bacterial infections; in this regard, urine culture, vaginal probes and even blood samples must be collected. The risk of secondary bacterial pneumonia should not be ignored and antibacterial treatment should be administered without delay if bacterial sepsis is suspected. Intravenous ceftriaxone can be administered initially until the response of clear results(38). Other authors reported to use cefaclor, cefotiam hydrochloride, ornidazole or ceftazidime, with satisfactory results(45).
Regarding corticosteroid treatment, it should be customized for each patient separately, according to the gestational age or the need of fetal maturity in preterm delivery. It may predispose to maternal hyperglycemia and maintain the replication of the virus in pulmonary epithelial cells(46). When preterm delivery is anticipated, in threatened preterm labor, the administration of betamethasone or dexamethasone is indicated for fetal lung maturity, and even methylprednisolone has been used in order to alleviate dyspnea and hypoxemia(38). The fetal benefit must be thus carefully evaluated compared to maternal risk, as some reports declare that corticosteroid use may worsen the outcome in SARS-CoV-2 infected patients, yet with inconclusive result as the dosage use for these patient was considerably higher than the glucocorticoid treatment used for fetal lung maturity.
Severe cases should be managed in a negative-pressure isolation room in the ICU, along with the support of a multidisciplinary team, such as obstetricians, intensive care specialists, anesthetists, virologists, microbiologists, neonatologists and infectious disease specialists(2,47); if needed, preterm delivery should be considered in order to improve maternal state. For more severe complications that may include septic shock, acute kidney injury, and virus-induced cardiac injury, and as severe respiratory failure might occur in latter stages, based on previous experience with SARS and MERS in the past years, the limited literature suggests a potential role of extracorporeal membrane oxygenation(47). In order to improve maternal oxygenation of these cases, some authors consider delivery(48), but the final decision should be taken according to gestational age, maternal condition and fetal stability(36).
Admitted patients with suspected or confirmed SARS-CoV-2 infection benefit from standard weight-adjusted thromboprophylaxis. In high-risk cases, postpartum first doses of LMWH should be administrated as soon as possible after birth, and the prophylaxis should be maintained at least 10 days after discharge. In cases with ongoing morbidity, thromboprophilaxis should be extended for 6 weeks. The antithrombotic treatment has also antiviral properties.
NHS Blood and Transplant Service is recruiting people, no sooner than 28 days after recovery, for passive immunization therapy in patients on intensive care units, including pregnant women. Rajendran et al. reviewed five observational studies of convalescent plasma, but further controlled tests are needed.
Several patients were prescribed Lianhua Qingwen capsule, which is a commonly used Chinese medical preparation to treat viral influenza, and can be used efficiently for light fever, cough and fatigue(45).
Currently, there are no approved vaccines for the prevention of SARS-CoV-2 infection, although several vaccines are under development and they will not be available soon.
Even though social distancing is highly important during this pandemic period, health care services should not be limited, especially not for pregnancy evaluation. Virtual consultation represents an efficient evaluation method, but at least six face-to-face consultations must be conducted in pregnant patient with no coexisting medical comorbidities, increasing the number as needed. Every effort should be made to ensure that relevant blood and urine tests, vaginal probes and treatment prescriptions are made at each appointment and reevaluate if the next visit is medically necessary or it could be rescheduled; also, further information and advice could be given via telephone, e-mail or video consultation(49).
Triaging obstetric antenatal visits, for uncomplicated pregnancies, will thus lead to the following six appointments: initial visit and dating scan, 11-13+6 week scan accompanied by combined test or NIPT (not advisable to reschedule), 18-23 week anatomical scan, 28-week visit for discussing current health, fetal movements, mental well-being, verify blood pressure and offer advice and sources of further support and information, and repeat blood tests to screen for anemia, anti-D prophylaxis for Rh-negative women when needed, 32-week ultrasound fetal growth scan clinically indicated(50), discussing the results of investigations at 28 weeks and plans for birth, and the 40-week visit, giving information about options for prolonged pregnancy(51). The sonographic examinations in women at low obstetric risk will be rescheduled after 2-3 weeks in symptomatic and/or screen positive results for SARS-CoV-2. Note that patients with an increased risk of complications will need additional appointments or multidisciplinary care, as preeclampsia, gestational diabetes, endocrine disorders, cardiac or respiratory pathology and many other are often encountered during pregnancy.
Conclusions
Taking into account the prothrombotic state induced by pregnancy, the immunothrombosis and the general inflammatory response due to SARS-CoV-2 infection, we believe that prenatal care should be focused on the prevention of three pregnancy complications: premature preterm rupture of membranes, placental insufficiency, and thromboembolism.
Having all the studies, case reports and even unofficial reports on pregnant women published so far, it is still difficult to measure accurately the efficacy of any therapy used to treat COVID-19 in pregnancy. Pregnant women represent a uniquely vulnerable group in any infectious disease.
For clinician, the most affected are the triage and diagnostic decision-making, especially in settings without ready access to laboratory testing or when surge capacity has been exceeded. For managers of health care services, it is important for rapid forward planning in terms of procurement of supplies, readiness of human resources to staff beds at different intensities of care and generally ensuring the sustainability of the health care system through the peak and duration of the epidemic.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
1. Di Mauro G, Cristina S, Concetta R, Francesco R, Annalisa C. SARS-Cov-2 infection: response of human immune system and possible implications for the rapid test and treatment. Int Immunopharmacol. 2020; 84:106519.
2. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061-9.
3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020; 382(18):1708-20.
4. Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history. Laeknabladid. 2008; 94(11):737-45.
5. Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010; 303(15):1517-25.
6. Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PW, Ho LC, To WW, Lai ST. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004; 191(1):292-7.
7. Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China, 2020. China CDC Weekly. 2020; 2(8):113-22.
8. Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, Peng Z, Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China.
J Clin Virol. 2020; 127:104364.
9. Berger A. Th1 and Th2 responses: what are they? BMJ. 2000; 321(7258):424.
10. Dashraath P, Jeslyn WJ, Karen LM, Min LL, Sarah L, Biswas A, Choolani MA, Mattar C, Lin SL. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020; pii: S0002-9378(20)30343-4.
11. Poon LC, Yang H, Lee JC, Copel JA, Leung TY, Zhang Y, Chen D, Prefumo F. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020; 55(5):700-8.
12. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395(10226):809-15.
13. Hosier H, Farhadian S, Morotti RA, Deshmukh U, LuCulligan A, Campbelet KH, et al. First case of placental infection with SARS-CoV-2. medRxiv. 2020; 04.30.20083907, doi: https://doi.org/10.1101/2020.04.30.20083907.
14. Brătilă E, Vlădăreanu S. Comisia de Obstetrică-Ginecologie a Ministerului Sănătăţii Comisia de Neonatologie a Ministerului Sănătăţii. Metodologia privind naşterea la gravidele cu infecţie suspicionată/confirmată cu SARS-COV-2/COVID-19, preluarea, îngrijirea şi asistenţa medicală a nou-născutului. 2020, Apr 20.
15. WHO. Coronavirus disease 2019 (COVID-19) situation report. Available at: https://www.who.int/ emergencies/diseases/novel-coronavirus-2019/situation-reports
16. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am College Cardiol. 2020; 75(18):2352-71.
17. Mattiuzzi C, Lippi G. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Ann Transl Med. 2020 Feb; 8(3):48.
18. Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020; 120(5):876-8.
19. WHO. Coronavirus disease 2019 (COVID-19) Situation Report – 97. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200426-sitrep-97-covid-19.pdf?sfvrsn=d1c3e800_6
20. Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Inf Dis. 2020; pii: ciaa325.
21. CDC. Symptoms of Coronavirus. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
22. WHO. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. Interim Guidance. 2020 March 9. Available at: https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19-laboratory-2020.5-eng.pdf?sequence=1&isAllowed=y
23. Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, Geng B, Muenker MC, Moore AJ, Vogels CB, Petrone ME. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv. 2020 Apr 22, doi: https://doi.org/10.1101/2020.04.16.20067835
24. Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020; doi: 10.1002/jmv.25727.
25. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270-3.
26. Lee HK, Lee BH, Seok SH, Baek MW, Lee HY, Kim DJ, Na YR, Noh KJ, Park SH, Kumar DN, Kariwa H. Production of specific antibodies against SARS-coronavirus nucleocapsid protein without cross reactivity with human coronaviruses 229E and OC43. J Vet Sci. 2010; 11(2):165-7.
27. Meyer B, Drosten C, Müller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014; 194:175-83.
28. Adams ER, Anand R, Andersson MI, Auckland K, Baillie JK, Barnes E, Bell J, Berry T, Bibi S, Carroll M, Chinnakannan S. Antibody testing for COVID-19: A report from the National COVID Scientific Advisory Panel. medRxiv. 2020 May 7, doi: https://doi.org/10.1101/2020.04.15.20066407
29. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395(10226):809-15.
30. Sun G, Tang F, Peng M, Gao Y, Peng J, Xie H, Zhao Y, Jin Z. Clinical features and outcomes of pregnant women suspected of Coronavirus disease 2019. J Infect. 2020; pii: S0163-4453(20)30212-7.
31. Soldati G, Demi M, Smargiassi A, Inchingolo R, Demi L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev Respir Med. 2019; 13(2):163-72.
Articole din ediţiile anterioare
Miastenia gravis în sarcină – o abordare multidisciplinară
Miastenia gravis (MG) este o afecţiune autoimună care afectează în principal femeile tinere (în a doua sau a treia decadă de viaţă) şi se caracteri...
Sarcina cicatricială după operaţia cezariană – o continuă dilemă terapeutică. Serie de cazuri şi review al literaturii
Sarcina cicatricială după operaţie cezariană (CSP) este o tulburare iatrogenă care pune viaţa în pericol, cu o incidenţă tot mai mare, din cauza cr...
Sarcina implantată pe cicatricea operaţiei cezariene – management
Sarcina implantată pe cicatricea operaţiei cezariene este o complicaţie potenţială a sarcinii survenite la o femeie cu mai multe naşteri prin opera...
Managementul hemoragiei post-partum, în funcţie de cauza de bază
Hemoragia post-partum (HPP) continuă a fi o cauză importantă de deces la nivel mondial. HPP este definită în mod clasic ca o pierdere de sânge de 5...