We present the case of a 57-year-old woman diagnosed with metastatic breast cancer, with no comorbidities, who had severe hepatotoxicity (grade 4) after the first cycle with docetaxel, trastuzumab and pertuzumab. She had completely recovered after the symptomatic treatment and we decided to switch docetaxel to paclitaxel, without hepatic toxicity or other adverse events. After five cycles with paclitaxel, she continued with trastuzumab and pertuzumab for six months, until disease progression, followed by trastuzumab emtansine. During the treatment, we closely monitored the liver function and the serum transaminases remained in normal range after we stopped docetaxel.
A case report of fulminant hepatitis due to docetaxel in breast cancer
Hepatită fulminantă postchimioterapie cu docetaxel în cancerul de sân – prezentare de caz
First published: 31 mai 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/OnHe.59.2.2022.6539
Abstract
Rezumat
Prezentăm cazul unei femei de 57 de ani diagnosticată cu cancer mamar metastatic, fără comorbidităţi, care a avut hepatotoxicitate severă (gradul 4) după primul ciclu cu docetaxel, trastuzumab şi pertuzumab. Ea şi-a revenit complet după tratamentul simptomatic şi am decis să trecem de la docetaxel la paclitaxel, care nu are toxicitate hepatică sau alte efecte adverse întâlnite la docetaxel. După cinci cicluri cu paclitaxel, pacienta a continuat cu trastuzumab şi pertuzumab timp de şase luni, până la progresia bolii, urmate de trastuzumab emtansin. În timpul tratamentului, am monitorizat îndeaproape funcţia hepatică, iar transaminazele serice au rămas în interval normal după ce am oprit docetaxelul.
Background
In literature, there is an association between docetaxel and serum aminotransferase elevations in up to half of patients, but values greater than five times the upper limit of normal (ULN) occur in less than 2% of cases. Also, alkaline phosphatase elevations and mild bilirubin elevations may happen, but liver injury from docetaxel is rare. Case reports of severe acute hepatic necrosis due to docetaxel have been described. This can happen in a few days or weeks after the first or second administration of docetaxel(1).
Hepatotoxicity due to paclitaxel is infrequent in clinical studies. Elevations of transaminases, alkaline phosphatase and bilirubin have been described in about 5 to 20 percent of cases. Liver toxicity is not dose dependent(2).
Trastuzumab may produce side effects that usually occur at the first dose of administration, during or after the infusion(2).
Hepatic injury attributed to trastuzumab, which required the discontinuation of the drug, is reported in literature, but is rare(3).
For pertuzumab, the incidence of hepatic events was similar with placebo, when was added to standard chemotherapy(1).
Case presentation
We present the case of a 57-year-old woman who came to our hospital in August 2018 for a lump in the inner upper quadrant of the right breast and complained of back pain. She started investigations with mammography which showed a spiculated opacity in the right breast.
Her biopsy revealed invasive breast carcinoma, NST, G3, ER 25%, PGR-, HER2+, KI67 45%.
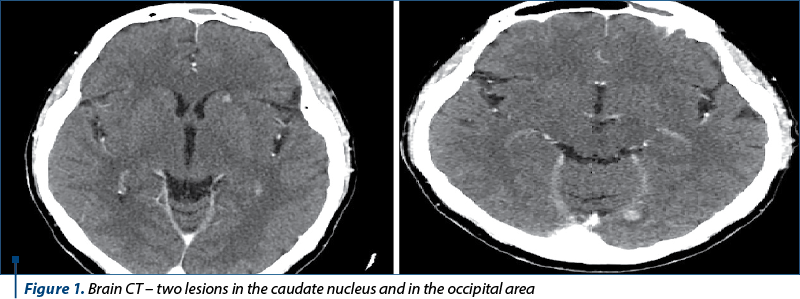
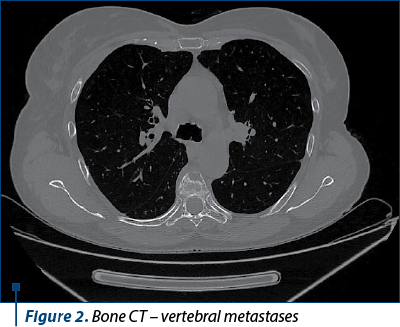
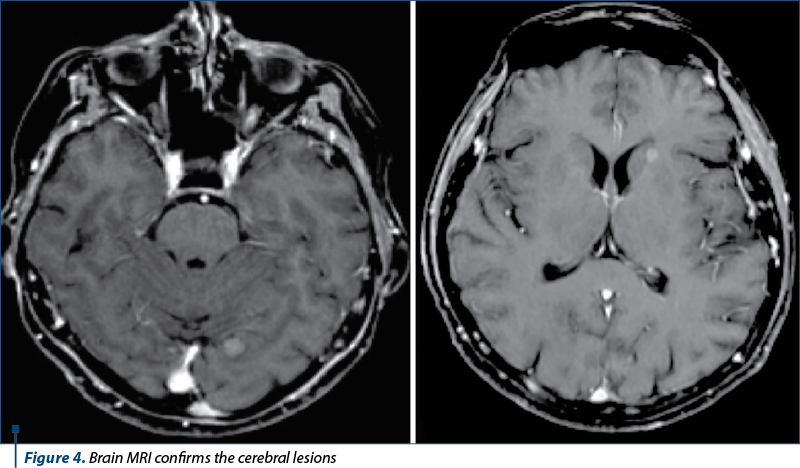
We continued the investigations with computed tomography (CT) of the brain, thorax, abdomen and pelvis, bone scintigraphy and brain magnetic resonance imaging (MRI).
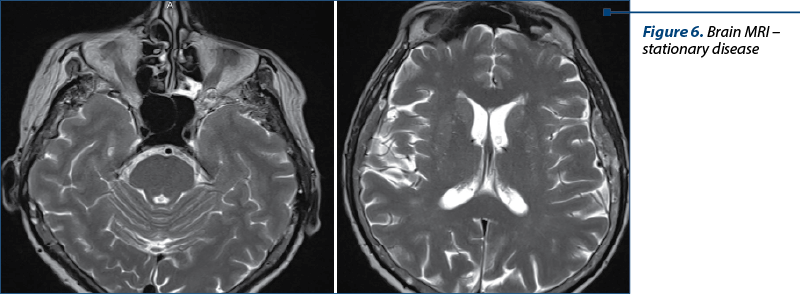
The diagnosis was stage IV breast cancer, T2N1, with asymptomatic brain and painful bone metastases.
We decided to start chemotherapy with docetaxel, pertuzumab, trastuzumab and zoledronic acid. At that point, all the biological analyses were normal. Also, the patient received whole brain radiotherapy in 10 fractions with a total dose of 30 Gy. After three weeks, she presented nausea, weakness, fatigue and back pain and the biological tests revealed ALT 821 U/L (27xULN), AST 340 U/L (19xULN), alkaline phosphatase 547 U/L, and mild hyperbilirubinemia (2.1 mg/dL). We suspected acute hepatitis after chemotherapy and the gastroenterology specialist recommended treatment with silymarin, arginine aspartate, acetylcysteine and granisetron, with improvements in symptomatology.
After two weeks, she came back for oncological treatment continuation, with good performance status (PS), asymptomatic, but ALT was still 8xULN and AST 6xULN. In this situation, we stopped docetaxel and pertuzumab and continued with trastuzumab and zoledronic acid, with careful monitorization of liver transaminases levels.
At the next cycle, the biological tests revealed ALT in normal range and AST slightly above normal limits (42 U/L), so we decided to change docetaxel with paclitaxel and continued trastuzumab, pertuzumab and zoledronic acid for five cycles. This modification was very well tolerated, without liver or other toxicities (serum transaminases remained in normal range).
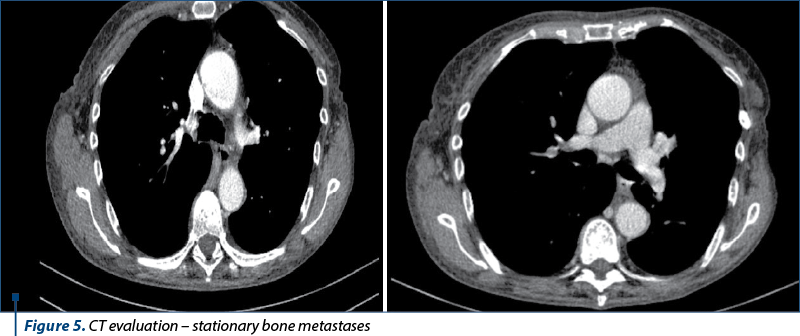
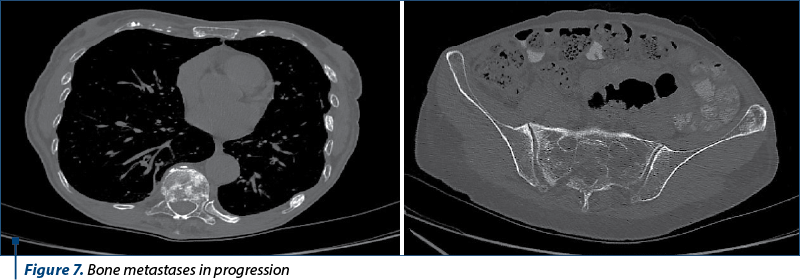
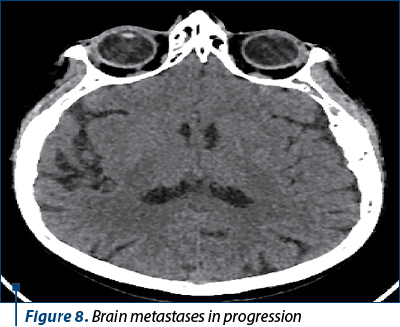
The next evaluation showed stable disease and the patient continued with the same treatment until the next CT evaluation, which showed progressive bone and brain metastases.
After disease evolution, we switched to trastuzumab emtansine, which was well tolerated and continued until February 2021, when the patient lost the follow-up.
Discussion
There was no comorbidity in this patient and her liver enzymes levels were normal at the initiation of treatment. The levels became elevated after the first cycle of treatment, and normalized after docetaxel discontinuation.
In literature, there are some cases of trastuzumab-induced hepatotoxicity, but this toxicity is rare(3).
We chose to continue trastuzumab after her transaminases were decreasing and after three more weeks the liver function returned to normal. We didn’t know if the hepatotoxicity was due to docetaxel, trastuzumab or pertuzumab, or the combination of the three agents, but it seemed more likely to be due to docetaxel.
Also, given the fact that docetaxel and paclitaxel belong to the same cytotoxic drug family, there was a theoretical risk of having the same adverse event after switching to paclitaxel, but our patient tolerated very well the additional five cycles of paclitaxel, without toxicity.
Docetaxel and paclitaxel are metabolized in the liver by the cytochrome p450 system, docetaxel predominantly by CYP3A4 and 3A5, and paclitaxel by CYP2C8 and 3A4, but the mechanism of injury is different. Docetaxel likely has a direct toxic effect on hepatocytes and paclitaxel has a direct effect in inhibiting microtubular function(1).
After the patient progressed on the first line and we had to change the treatment, we considered that trastuzumab emtansine was the best option. A meta-analysis based on this topic, written by A.M. Cobert, included a total number of 5045 patients, of whom 2893 received TDM1 and 2152 received other treatment. In the TDM1 arm, the incidence of all-grade transaminase elevations was as high as 43.5% for AST and 26.1% for ALT, and 11.3% and 9.2% for the control arm. The incidence of grade 3/4 AST and ALT elevation were 8.7% and 10.1%, respectively, for TDM-1 arm. In the control arm, the values were 0% for AST and 0.4% for ALT(4). Considering all these data, we still decided to start TDM1, but to monitor her liver function closely. The patient received this treatment for more than a year, without toxicity.
Conclusions
Liver function should be closely monitored in patients undergoing treatment with docetaxel. If the hepatic toxicity occurs, this doesn’t necessary mean that the patient will have the same toxicity after other microtubule inhibitors (including paclitaxel, trastuzumab emtansine or nab-paclitaxel).
Conflict of interests: The authors declare no conflict of interests.

Bibliografie
-
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Docusate. [Updated 2018 Jan 9].
-
Mandaliya H, Baghi P, Prawira A, George MK. A Rare Case of Paclitaxel and/or Trastuzumab Induced Acute Hepatic Necrosis. Case Rep Oncol Med. 2015;2015:825603. doi:10.1155/2015/825603.
-
Srinivasan S, Parsa V, Liu CY, Fontana JA. Trastuzumab-induced hepatotoxicity. Ann Pharmacother. 2008;42(10):1497-1501. doi:10.1345/aph.1L217.
-
Cobert AM, Helms C, Larck C, Moore DC. Risk of hepatotoxicity with trastuzumab emtansine in breast cancer patients: a systematic review and meta-analysis. Therapeutic Advances in Drug Safety. January 2020. doi:10.1177/2042098620915058.
Articole din ediţiile anterioare
Faslodex in second line of hormonal treatment of advanced breast cancer - case report
Acest caz se referă la o pacientă cu cancer mamar avansat local şi cu metastaze osoase multiple. Pacienta a suferit o intervenţie chirurgicală pali...
Metaplastic breast cancer. German guidelines for triple-negative breast carcinoma
Introducere. Carcinomul mamar metaplastic este un subtip agresiv de carcinom mamar invaziv, rar, cu o incidenţă mai mică de 1%, caracterizat printr...
Can we achieve successfully local control by IMRT radiotherapy in the treatment of male breast cancer? A case report
Această prezentare descrie un caz rar de cancer mamar la un pacient de gen masculin, patologie care are o incidenţă mai mică de 1% din toate cazur...
Evaluarea cardiotoxicităţii din timpul tratamentului cancerului de sân - actualizare
Tratamentul cancerului de sân cuprinde o varietate de agenţi chimioterapici, de la clasicele citostatice, precum antraciclinele, ciclofosfamida, ta...