Chronic rhinitis is a frequent affliction, with a significant impact on the patients’ quality of life. Materials and method. Surgically harvested samples from the inferior turbinate of 60 patients presenting with hypertrophy of the inferior nasal turbinate were included in paraffin blocks, sectioned stained and analyzed, with the aim to examine cellular pattern and the physiopathological changes that occur in the nasal mucosa of patients suffering from nonallergic noninfectious chronic rhinitis. The parameters observed were: the aspect of the basal membrane, the presence of local edema, the presence of local hyperemia, the presence or absence of hematic extravasations, the degree of infiltration, the number of lymphocytes, plasma cells, eosinophils, the location of the inflammatory infiltrate and the presence of fibrosis. Results. The results of histologic and statistical analysis showed basal membrane thickening in 70% of cases, significant mucosal hyperemia in 66.6% of cases, and blood extravasations in 51.6%. Tissue edema was negligible, being found in only 7% of samples, a major difference compared with allergic rhinitis. All tissue samples had inflammatory infiltrate, but in 41.6% there was a minimal infiltrate. The majority of cell types in the inflammatory infiltrate of chorion and nasal epithelium were lymphocytes (55%) and just a few eosinophils. In about 93% of cases, chorion fibrosis was observed, from which 35% had important fibrosis, considered a remodeling process and interpreted as a tendency of progression toward persistent or irreversible mucosal changes. Conclusions. Correlation strength between the expression of clusters of differentiation CD20, CD3, CD11c and CD68c, markers for the presence and activity of different cell types and the inflammatory infiltrate, respectively fibrosis in chorion and nasal epithelium, lead to the conclusions that neither humoral immunity, nor cellular immunity seem to be correlated with inflammatory infiltrate of nasal mucosa. Increased CD68 expressions in relation with important inflammatory infiltrate or fibrosis may signify a potential role of macrophages in infiltrate development, an increased nonspecific phagocytosis, and they could be a marker of remodeling of nasal mucosa in chronic rhinitis.
Histological findings in chronic hypertrophic rhinitis
Modificări histologice în rinita hipertrofică
First published: 24 noiembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/ORL.61.4.2023.8960
Abstract
Rezumat
Rinita cronică este o afecţiune frecventă, cu un impact semnificativ asupra calităţii vieţii. Scopul lucrării îl reprezintă examinarea modelului celular şi modificările fiziopatologice care apar în mucoasa nazală a pacienţilor cu rinită cronică noninfecţioasă nonalergică. Materiale şi metodă. Probele recoltate chirurgical din cornetul nazal inferior de la 60 de pacienţi care prezentau hipertrofie a cornetelor nazale inferioare au fost incluse în blocuri de parafină, colorate şi analizate. Parametrii observaţi au fost: aspectul membranei bazale, prezenţa edemului local, prezenţa hiperemiei locale, prezenţa sau absenţa extravazărilor hematice, gradul de infiltraţie, numărul de limfocite, plasmocite, eozinofile, localizarea infiltratului inflamator şi prezenţa fibrozei. Rezultate. Rezultatele analizelor histologice şi statistice au arătat îngroşarea membranei bazale în 70% din cazuri, hiperemie semnificativă a mucoasei în 66,6% din cazuri şi extravazări hematice la 51,6%. Edemul tisular a fost neglijabil, s-a constatat la doar 7% din probe, o diferenţă majoră faţă de rinita alergică. Toate probele de ţesut au avut infiltrat inflamator, dar în 41,6% din cazuri infiltratul a fost minim. Majoritatea tipurilor de celule din infiltratul inflamator al corionului şi al epiteliului nazal au fost limfocite (55%) şi doar câteva eozinofile. În aproximativ 93% din cazuri, s-a observat fibroză la nivelul corionului, dintre care 35% au avut fibroză semnificativă, considerată un proces de remodelare şi interpretată ca o tendinţă de progresie spre modificări persistente sau ireversibile ale mucoasei. Concluzii. Corelaţia dintre expresia clusterelor de diferenţiere CD20, CD3, CD11c şi CD68c, markeri pentru prezenţa şi activitatea diferitelor tipuri de celule şi infiltratul inflamator, respectiv fibroza în corion şi epiteliul nazal, conduc la concluzia că nici imunitatea umorală şi nici imunitatea celulară nu par a fi corelate cu infiltratul inflamator al mucoasei nazale. Expresiile crescute ale CD68 în relaţie cu infiltratul inflamator important sau cu fibroza pot însemna un rol potenţial al macrofagelor în apariţia infiltratului, o fagocitoză nespecifică crescută, şi ar putea fi un marker al remodelării mucoasei nazale în rinita cronică.
Introduction
Chronic rhinitis is one of the commonest conditions diagnosed in the ENT service, based on symptoms and physical signs.
A significant number of patients suffer from persistent rhinitis that impacts their quality of life, generates recurrent visits to ENT specialists and often the use of self-administered topic treatments, leading to mucosal damage. Allergic rhinitis and infectious or polypoid rhinosinusitis were the subject of many studies, but there are only a few references about nonallergic noninfectious chronic rhinitis, despite its frequency in clinical practice.
The purpose of this study was to examine and characterize the cellular pattern and the physiopathological changes that occur in the nasal mucosa of patients suffering from nonallergic noninfectious chronic rhinitis. Histopathology offers meaningful information about changes that occur in the nasal mucosa of patients with hypertrophic rhinitis.
Materials and method
To carry out our anatomopathological and immunohistochemical study, 60 cases presenting with hypertrophy of the inferior nasal turbinate were analyzed, spanning over a three-year interval. The samples were obtained surgically, in the Otorhinolaryngology Department of the “Prof. Dr. Dorin Hociotă” Institute of Phonoaudiology and Functional Surgery, Bucharest, Romania. Biopsies were processed in the anatomopathological department of the Colentina Clinical Hospital, Bucharest.
All patients agreed that their medical data could be used in scientific studies.
Inclusion criteria: we included in the study patients presenting with persistent specific symptomatology of chronic rhinitis, regardless of age or gender (persistent nasal obstruction, anterior or posterior rhinorrhea, decreased smell, itchy nose etc.).
The main exclusion criteria were: the presence of associated local tumors, the presence of acute sinusitis or rhinitis, allergic rhinitis and rhinosinusitis with or without polypoid degeneration, neoplasms or life-threatening chronic diseases, patients who underwent nasal surgery that could modify the physiology of nasal mucosa.
A detailed anamnesis was performed in order to correctly classify the pathology. The patients were asked to complete a validated questionnaire that measured the physical, functional and emotional impact of the patient diagnosed with rhinitis and rhinosinusitis. The chosen questionnaire was the Rhinosinusitis Disability Index, which is used to validate the impact of the disease on the quality of life in the European Union.
The endoscopic examination of the nasal cavity evaluated the presence and degree of nasal pathology using the Lund-Kennedy endoscopic scoring system.
Biologic material
Biopsies were harvested from the inferior turbinate under anesthesia and rigid endoscopic guidance, and immediately placed in 10% buffered formalin. Histopathological processing consisted of dehydration, clarification and impregnation with paraffin by automated processing. The paraffin blocks were sectioned and the sections were stained with routine staining (hematoxylin eosin – HE) or immunohistochemically (IHC), respectively.
Paraffin-impregnated sections were embedded in paraffin blocks on the Leica EG 1150H paraffin embedding station and sectioned at 3 µ on Leica RM 2255 and RM 2265 microtomes. The sections were stained with routine staining (hematoxylin eosin) or immunohistochemistry (IHC). HE staining was performed with the Leica ST 5020 automatic staining station and Leica CV 5030 automatic mounter.
Several primary antibodies were used. Secondary antibodies and streptavidin complex from the Novolink kit (Leica), as well as liquid DAB chromogen (Leica) were used for development. IHC staining were performed either manually or with the automatic Leica Bond III immunostainer. The recipes for HE staining and IHC staining comply with the provisions of the medical practice guidelines for the pathological anatomy specialty.
Statistical analysis
Data were statistically processed and statistically analyzed with the help of the Microsoft Office Excel 2019 program. For the statistical analysis, we used the c2 test, the level of statistical significance being p<0.05. Chi-square test was utilized to reveal the significance of the correlation between different histological findings. Because the Chi-square test can only state whether a link between variables is significant or not, the Phi coefficient and Cramér’s V, that shows the intensity of the link between variables (the values of the coefficient are in the range [-1 – 1])(1), were used.
Parameters
The markers analyzed were: the aspect of the basal membrane, the degree of local edema, the degree of local hyperemia, the presence or absence of hematic extravasations, the degree of infiltration, the number of lymphocytes, the number of plasma cells, the number of eosinophils, the location of the inflammatory infiltrate, and the degree of fibrosis. We also studied the presence of CD20, CD3, CD11c and CD68c markers in the chorion and intraepithelial.
Results
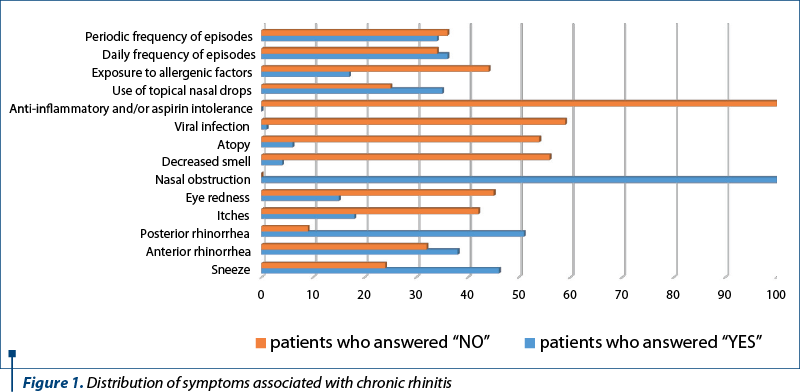
All 60 patients included in the study presented nasal obstruction as main symptom that brought them to the doctor. Most patients presented nasal obstruction all year round, with little influence by physical activity, position, administered drugs or environmental factors.
The dominant age range was 35 to 54 years old (more than 40%). It is worth mentioning that 58% of patients included in the study were chronic users of vasoconstrictor nasal drops in order to improve their nasal obstruction, a habit that threw them into a vicious circle of vasoconstrictors dependency.
Sixty-five percent of patients complained of posterior rhinorrhea. Because allergic rhinitis was excluded, only a few patients complained of loss of smell, itchy nose and anti-inflammatory drugs intolerance.
The majority of patients who addressed the ENT department and were diagnosed with chronic rhinitis presented grade III hypertrophy (about 60%) of the inferior nasal turbinates, but only 10% associated middle turbinate hypertrophy. Ninety percent of patients had bilateral changes. Unilateral changes correlated with contralateral septal deviation, a significant septal crest or spur, possibly as a compensatory change.
Basal membrane thickening was noticed in 70% of cases. The basal membrane of the biopsied inferior nasal turbinate was most frequently diffusely thickened – 51.66% of performed examinations. A percentage of 18.33% had irregular thickening and 26.66% showed a normal size of the inferior nasal turbinate basal membrane.
The anatomopathological examination of biopsy samples taken from patients diagnosed with chronic rhinitis revealed the existence of significant tissue edema in only 7% of cases, the other 93% didn’t have edematous degeneration of nasal mucosa.
Moreover, 76.66% of the biopsy samples showed mucosal hyperemia (significant in 66.66% and minimal in 10% of cases), and 23.33% of cases did not present the appearance of hyperemia at all. We can conclude that increased afflux of blood is a contributor to nasal obstruction described by patients, beside inflammatory infiltrate and chorion fibrosis.
Also, 51.66% of the biopsy samples taken revealed the presence of blood extravasations on anatomopathological examination and a percentage of 48.33% had absent blood extravasations. The association between blood extravasation and the significant inflammatory infiltrate supports the fact that interstitial hemorrhage exists in the inflammatory context, and that the severity of the inflammation favors blood suffusions.
In 50% of the cases, inflammatory infiltrate was found at the interstitial level, in 25% of samples being perivascular and periglandular. The periglandular area was the second most frequent infiltrate location after the interstitial one. In 11.66% of cases, there was diffusely located infiltrate. The perivascular and vascular inflammation registered the least percentage, so a vasculitis component was excluded as a potential cause of hypertrophy in the study group.
In 93% of cases, fibrosis was noted, but in 58.33% it was minimal. An important fibrosis was observed in 35% of the analyzed cases, and in 3.33% of cases, the fibrosis was absent. Also, in 3.33% of cases, the appearance of fibrosis looked reactive. We may consider fibrosis a remodeling process and relate it to the degree of hypertrophy and time span of symptoms.
Regardless the degree of inflammatory infiltrate of mucosa taken from the inferior nasal turbinate (either small or important), just a few eosinophils were found in the chorion and nasal epithelium. Fifty-five percent of biopsy samples revealed the presence of a large number of lymphocytes on the anatomopathological exam.
We found no correlations neither between the chorion and epithelial infiltrate and CD11c expression, nor between fibrosis and intraepithelial CD11c expression, but there was a significant correspondence between fibrosis and CD11c in the chorion. The similar density of dendritic cells deduced from the similar expression of CD11c regardless de degree of inflammation made us think that antigen presenting cells are not significant in chronic rhinitis and they are not associated with disease severity. Their presence at the chorion level and less at the epithelium, in correlation with fibrosis, seems rather related to remodeling, chronicity and progress towards irreversibility.
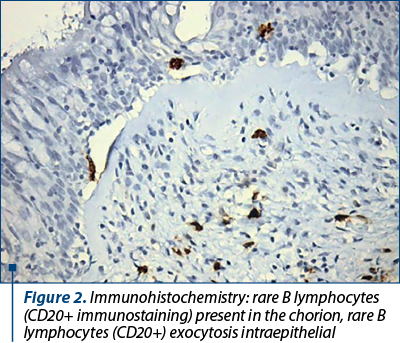
B lymphocytes don’t seem related to the density of the inflammatory infiltrate, making improbable the hypothesis of an exogenous pathogen present at the time of the biopsy. The difference between the presence of B lymphocytes in the chorion and the intraepithelial extravasation may indicate that severe chronic inflammatory process once established can be self-maintaining. The remark that B CD20+ lymphocytes were present in a higher percentage in cases with significant fibrosis and fibrosis is related to injury progression towards irreversibility, drawing attention to a role that humoral immunity component may play in irreversible processes.
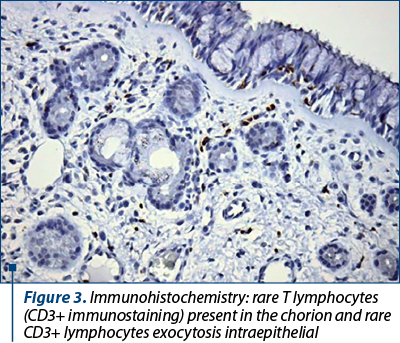
We found no correlations between the chorion and epithelial infiltrate and CD3 expression, meaning that exocytosis of T lymphocytes does not relate to inflammation severity, underlining the reduced importance of the cellular immune component in chronic rhinitis among the patients in the studied group. CD3 expression was more than expected in samples with fibrosis, indicating the intervention of the cellular immune component in the irreversibility of the chronic inflammatory process.
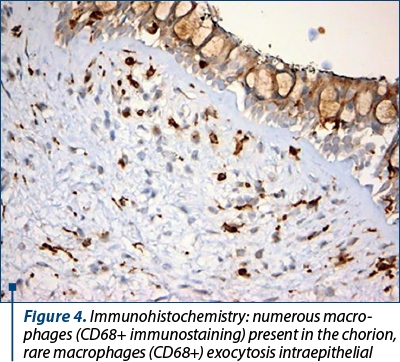
The fact that we found a correlation between CD68 expressions and inflammatory infiltrate may signify either an involvement in infiltrate development or an increased nonspecific phagocytosis in cases with abundant inflammatory infiltrate. According to the 0.51 value (Phi coefficient and Cramér’s V), it is considered that the link between the infiltrate and the CD68 chorion is a very strong one. We considered increased CD68 expression found to be correlated with chorion fibrosis (strong relation according to Phi coefficient and Cramér’s V of 0.39), a marker of remodeling of nasal mucosa in chronic rhinitis.
Discussion
Rhinitis is defined as an inflammatory process of allergic and nonallergic cause that affects nasal mucosa, associated with an accumulation of inflammatory cells in the nasal cavity lumen and with structural changes(2). Although a common disease, it significantly impacts the patient’s quality of life.
The respiratory nasal mucosa is a component of innate immunity, a physical barrier against pathogenic respiratory agents, allergens, physical insults or other potentially threating agents. It has an important role in maintaining a balance between commensal microbiome and pathogenic agents inhaled through the nose, its dysfunctions leading to common diseases, with a significant impact on patient’s quality of life(3,4).
The mucosa of posterior two-thirds of the cavity is a pseudostratified columnar ciliated epithelium containing goblet cells that produce mucus, overlying a basement membrane. The basal membrane contains glands which secrete watery substances and mucus, nerves, an extensive network of blood vessels and cellular elements like blood plasma(6).
High density of blood vessels and large venous-like spaces have been described in nasal mucosa. In response to infection or allergy or other causes, these vascular bodies, which have a vein-like appearance, swell and congest. The mucus secreted by the mucosa traps the pathogens that enter nasal cavity and, through the ciliary movement, drives them toward de pharynx(5-7).
The respiratory nasal mucosa also mediates local and systemic inflammatory responses to a wide range of pathogens(8).
While the allergic rhinitis and polypoid chronic rhinosinusitis have been of great interest in literature, histological changes that characterize nonallergic rhinitis are poorly represented. This study tried to examine cellular patterns and the physiopathological changes that occur in the nasal mucosa of patients suffering from nonallergic noninfectious chronic rhinitis.
Thickened basal membrane was described as part of a remodeling process that takes place in allergic rhinitis and chronic rhinosinusitis in correlation with an increased number and activity of eosinophils(9,10). In 70% of our samples from inferior turbinate of nonallergic patients, thickening of basal membrane was also present (diffuse or irregular), without an increased number of eosinophils in the mucosa.
Tissue edema was negligible, being found in only 7% of samples, a major difference compared with allergic rhinitis. Allergic pathology – especially polypoid rhinosinusitis – is characterized by stromal tissue edema, beside inflammatory cell infiltrate with a high number of eosinophils(11).
All tissue samples had inflammatory infiltrate, but 41.6% had only minimal infiltrate. The predominant cell type in the inflammatory infiltrate of both chorion and nasal epithelium was represented by lymphocytes (55%), in contrast with just a few eosinophils, another important difference from allergic rhinitis.
The histological findings of nasal mucosa in allergic rhinitis revealed an increased infiltration of eosinophils and mast cells in the submucosal layer(12). According to other studies, the increase in the number of nasal eosinophils showed a better correlation with the nasal obstruction symptom in patients with persistent allergic rhinitis(13).
In perennial rhinitis, the accumulation of eosinophils and mast cells was described and correlated with the loss of epithelial integrity in the nasal mucosa(14). In chronic rhinosinusitis, epithelial hyperplasia, increased matrix deposition and degradation along with accumulation of plasma proteins were described by other authors(8,15).
In order to explore the presence and activity of different cell types in chorion and nasal epithelium, the present study used CD (clusters of differentiation) as markers. Clusters of differentiation are cell surface molecules with various roles that can serve to identify cells with certain immune functions(16).
We analyzed the correlation strength between the expression of CD20, CD3, CD11c and CD68c in the chorion and epithelium and the inflammatory infiltrate and fibrosis, respectively, in the nasal mucosa. The main remarks drawn from the results strengthened the differences between allergic and nonallergic rhinitis.
CD11c is a widely established marker for dendritic cells. It is a transmembrane protein that is expressed also on monocytes, granulocytes, a subset of B cells and macrophages. Dendritic cells are antigen-presenting cells; they process antigen material and present it to T lymphocytes, acting as messengers between the innate and the adaptive immune systems(17).
Dendritic cells have a pivotal role in immune homeostasis, marshaling immunity against pathogens, while maintaining tolerance to commensals(18).
Antigen presenting cells (mostly for T lymphocytes) proved not to be significant in chronic rhinitis and can’t be associated with disease severity, but they may be related to remodeling and chronicity.
T lymphocytes do not relate to inflammation severity, underlining the reduced importance of the cellular immune component in chronic rhinitis.
CD20 was used to assess the presence and activity of B cells, key constituent of the adaptive immune response, in the chorion and epithelium of nasal mucosa samples(19). It is a transmembrane protein found on B cells that forms a calcium channel allowing for the influx of calcium required for cell activation. CD20 is expressed on almost all stages of B cell development, but not in plasmocytes(20,21).
B cells can also act as antigen-presenting cells or regulatory cells by producing a large array of cytokines that influence the inflammatory response(22). CD20 is the target of monoclonal antibodies therapies on B cell neoplasia and B cell mediated immune diseases(22).
CD20 is an antigen involved in modulation, proliferation and differentiation of B cells(23). By analyzing its expression in the chorion and nasal epithelium, we have concluded that B lymphocytes don’t seem related to the density of the inflammatory infiltrate.
CD3 (cluster of differentiation 3) is a protein complex and T cell coreceptor useful in generating an activation signal in T lymphocytes. It is a useful immune-histochemical marker for T-cells presence and activity in tissue sections(24).
T cells are lymphocytes responsible for cellular immunity. B cells have the ability to transform into plasmocytes that produce antibodies. Thus, humoral immunity depends on the B cells, while cell immunity depends on the T cells(25,26).
CD68 is a transmembrane glycoprotein used as immune-cytochemical marker for monocytes and macrophages. Macrophages are phagocytes that engulfs and digests pathogens, dead neutrophils and cellular debris, thus playing an important role in innate immunity(27).
They are also antigen presenting cells for T lymphocytes. Along with dendritic cells, they secrete cytokines, promote inflammation through M1 population or have anti-inflammatory effect and promote tissue repair through M2 population(28).
Macrophages’ involvement in promoting tissue repair is beyond debriding damaged tissue; they also secrete a number of factors that increase healing(29,30).
Increased CD68 expressions in correlation with significant inflammatory infiltrate or significant fibrosis can be explained by a potential role of macrophages in infiltrate development, an increased nonspecific phagocytosis, or they could be a marker of remodeling of nasal mucosa in chronic rhinitis.
In inflammatory diseases, a process of remodeling has been described, leading to either a normal or a pathological reconstruction process(31,32). In a histological study, Watelet et al. characterized the remodeling process in rhinitis as increased thickness, epithelial detachment and pseudofibrosis of the basement membrane(31).
In about 93% of samples analyzed, chorion fibrosis was observed, 35% representing important fibrosis. This fact was considered a remodeling process and interpreted as a tendency of progression toward persistent or irreversible mucosal changes.
Looking to histological characteristics of nasal mucosa in cases of chronic rhinitis is one step closer to understanding this pathology and to applying an evidence-based treatment. Many times, this annoying pathology for patients goes through many time-consuming attempts of treatment. A deeper understanding may hopefully lead to new ideas of therapeutic tools or strategies to improve the patients’ life.
Conclusions
The aim of this study was to examine the cellular patterns and the physiopathological changes in the nasal mucosa of patients with nonallergic noninfectious chronic rhinitis. The histopathological analysis of surgically obtained samples from the inferior turbinate revealed several important findings. Basal membrane thickening was observed in the majority of cases, along with significant mucosal hyperemia and blood extravasations. Tissue edema was minimal, unlike in allergic rhinitis. Inflammatory infiltrate was present in all samples, primarily consisting of lymphocytes and a few eosinophils. Fibrosis in the chorion was observed in the majority of cases, with a significant proportion showing important fibrosis, indicating a remodeling process and potential progression toward persistent or irreversible mucosal changes.
Interestingly, no significant correlations were found between the expression of specific cell markers and the inflammatory infiltrate or fibrosis, suggesting that neither humoral, nor cellular immunity play a significant role in chronic rhinitis. However, increased CD68 expression was associated with important inflammatory infiltrate and fibrosis, indicating a potential role of macrophages in the infiltrate development and remodeling of nasal mucosa.
These findings shed light on the histological characteristics of nonallergic noninfectious chronic rhinitis and provide insights into the pathophysiological processes involved. Understanding these changes can contribute to a better management and treatment strategies for patients suffering from this condition. Further research is warranted to explore the underlying mechanisms and the potential therapeutic targets in chronic rhinitis.
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
-
McHugh ML, User’s guide to correlation coefficient.Turkish Journal of Emergency Medicine. Haldun Akoglu, 2018.
-
Vlaminck S, Prokopakis E, Kawauchi H, Haspeslagh M, Van Huysse J, Simões J, Acke F, Gevaert P. Proposal for Structured Histopathology of Nasal Secretions for Endotyping Chronic Rhinosinusitis: An Exploratory Study. Allergies. 2022;2(4):128-137.
-
Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021;14(2):305-316.
-
Harvey RJ, Lund VJ. Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology. 2007;45(1):3-13.
-
Marieb EN. Essential of Human Anatomy and Physiology, 11th Edition. Chapter 13. The Respiratory System. San Francisco: Benjamin Cummings; 2018, pp. 801-847.
-
Orahilly R, Muller F, Carpenter S, Swenson R. Chapter 52. The nose and paranasal sinuses. In: Swenson R (Ed.). Basic Human Anatomy. Dartmouth Medical School. 2008.
-
Lane AP. Nasal anatomy and physiology. Facial Plast Surg Clin North Am. 2004;12(4):387-95.
-
Jeican II, Gheban D, Barbu-Tudoran L, et al. Respiratory Nasal Mucosa in Chronic Rhinosinusitis with Nasal Polyps versus COVID-19: Histopathology, Electron Microscopy Analysis and Assessing of Tissue Interleukin-33. J Clin Med. 2021;10(18):4110.
-
Kovalhuk LC, Telles EQ, Lima MN, Filho NA. Nasal lavage cytology and mucosal histopathological alterations in patients with rhinitis. Braz J Otorhinolaryngol. 2020;86(4):434-42.
-
Samitas K, Carter A, Kariyawasam HH, Xanthou G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy. 2018;73(5):993-1002.
-
Stevens WW, Schleimer RP, Chandra RK, Peters AT. Biology of nasal polyposis. J Allergy Clin Immunol. 2014;133(5):1503-1503.e15034.
-
Goh HJ. The Histological Observation of Nasal Mucosa in Allergic Rhinitis of Positive Reaction with Allergen Skin. Korean Journal of Otorhinolaryngology - Head and Neck Surgery. 1983;26(1):38-42.
-
Ciprandi G, Vizzaccaro A, Cirillo I, Tosca M, Massolo A, Passalacqua G. Nasal eosinophils display the best correlation with symptoms, pulmonary function and inflammation in allergic rhinitis. Int Arch Allergy Immunol. 2005;136(3):266-272.
-
Amin K, Rinne J, Haahtela T, et al. Inflammatory cell and epithelial characteristics of perennial allergic and nonallergic rhinitis with a symptom history of 1 to 3 years’ duration. J Allergy Clin Immunol. 2001;107(2):249-257.
-
Samitas K, Carter A, Kariyawasam HH, Xanthou G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy. 2018;73(5):993-1002.
-
Zola H, Swart B, Banham A, Barry S, Beare A, Bensussan A, Boumsell L, Buckley C, Bühring HJ, Clark G, Engel P, Fox D, Jin BQ, Macardle PJ, Malavasi F, Mason D, Stockinger H, Yang X. CD molecules 2006 – human cell differentiation molecules. J Immunol Methods. 2007;319(1-2):1-5.
-
Monga I, Kaur K, Dhanda SK. Revisiting hematopoiesis: applications of the bulk and single-cell transcriptomics dissecting transcriptional heterogeneity in hematopoietic stem cells. Brief Funct Genomics. 2022;21(3):159-176.
-
Lee H, Ruane D, Law K, et al. Phenotype and function of nasal dendritic cells. Mucosal Immunol. 2015;8(5):1083-1098.
-
Mitroi M, Albulescu D, Capitanescu A, et al. Differences in the distribution of CD20, CD3, CD34 and CD45RO in nasal mucosa and polyps from patients with chronic rhinosinusitis. Mol Med Rep. 2019;19(4):2792-2800.
-
Walport M, Murphy K, Janeway C, Travers PJ. Janeway’s Immunobiology (7th Ed.). New York: Garland Science, 2008.
-
Hardy R. Chapter 7: B Lymphocyte Development and Biology. In: William P (Ed.). Fundamental Immunology (6th Ed.). Philadelphia: Lippincott Williams & Wilkins. 2008; pp. 237–269.
-
Ickrath P, Kleinsasser N, Ding X, Ginzkey C, Beyersdorf N, Kerkau T, Hagen R, Hackenberg S. Impact and modulations of peripheral and edaphic B cell subpopulations in chronic rhinosinusitis with nasal polyposis. Clin Exp Otorhinolaryngol. 2018;11(2):133–140.
-
Pavlasova G, Mraz M. The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica. 2020 Jun;105(6):1494-1506.
-
Zheng L, Lin J, Zhang B, Zhu Y, Li N, Xie S, et al. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. 2019;573(7775):546–552.
-
Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2(5):336–45.
-
Cano RLE, Lopera HDE. Introduction to T and B lymphocytes. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al. (editors). Autoimmunity: From Bench to Bedside. Bogota (Colombia): El Rosario University Press; 2013. Chapter 5. https://www.ncbi.nlm.nih.gov/books/NBK459471/.
-
Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32(6):463-488.
-
Murphy K, Weaver C. Janeway’s immunobiology. Garland Science, New York. 2006; pp. 464, 904.
-
Deodhar AK, Rana RE. Surgical physiology of wound healing: a review. Journal of Postgraduate Medicine. 1997;43(2):52-56.
-
Kunz-Schughart LA, Weber A, Rehli M, et al. Der “klassische” Makrophagenmarker CD68 ist in primären humanen Fibroblasten stark exprimiert [The “classical” macrophage marker CD68 is strongly expressed in primary human fibroblasts]. Verh Dtsch Ges Pathol. 2003;87:215-223.
-
Watelet JB, Van Zele T, Gjomarkaj M, et al. Tissue remodelling in upper airways: where is the link with lower airway remodelling?. Allergy. 2006;61(11):1249-1258.
-
Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720-45.
Articole din ediţiile anterioare
Hipertrofia cornetelor nazale inferioare: comparaţie a tehnicilor chirurgicale prin evaluarea complicaţiilor postoperatoriu
Obstrucţia nazală este unul dintre simptomele cel mai frecvent raportate în practica clinică. A doua cea mai frecventă cauză a obstrucţiei nazale...