Type 1 diabetes mellitus (T1DM) is an impressive autoimmune disease with the potential for lifelong complications. From life-threatening acute complications to chronic complications, most of which are expressed in adulthood, all of these affect the patients' quality of life. The pathogenesis of type 1 diabetes mellitus is still incompletely elucidated. Autoimmunity is obviously involved, the triggers are epidemiologically identified, but there are individual peculiarities of onset, diagnosis, treatment, follow-up and progression. Also, the involvement of T1DM in systemic pathologies is very common, especially towards adulthood.
Actualităţi în etiopatogenia diabetului zaharat de tip 1 la copil
Update on the etiopathogenesis of type 1 diabetes mellitus in children
First published: 19 decembrie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.72.4.2023.9275
Abstract
Rezumat
Diabetul zaharat de tip 1 (DZ tip 1) reprezintă o patologie autoimună impresionantă prin potenţialul complicaţiilor pe care le poate induce de-a lungul vieţii. De la complicaţii acute ameninţătoare de viaţă până la complicaţii cronice, majoritatea cu expresie la vârsta adultă, toate acestea afectează calitatea vieţii pacienţilor. Patogenia diabetului zaharat de tip 1 este încă incomplet elucidată. Autoimunitatea este evident implicată, factorii declanşatori sunt epidemiologic identificaţi, dar există particularităţi individuale de debut, diagnostic, tratament, urmărire şi evoluţie. De asemenea, implicarea DZ tip 1 în patologiile sistemice este foarte frecventă, mai ales spre vârsta de adult.
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic disease characterized by the body’s inability to produce insulin due to autoimmune destruction of beta cells in the pancreas. Most pediatric patients with diabetes have type 1 diabetes and a lifelong dependence on exogenous insulin.
In general, type 1 diabetes starts with acute symptoms and high blood glucose levels, with most cases being diagnosed soon after the onset of hyperglycemia. Comprehensive testing for specific autoantibodies in all asymptomatic patients cannot be recommended at present as a means of screening patients at risk. These tests may be appropriate in high-risk individuals – i.e., history of transient hyperglycemia, heredocolateral history of type 1 diabetes.
The diagnosis of T1DM at pediatric age has extraordinary lifelong consequences in terms of lifestyle, quality of life, and the appearance sooner or later of complications that can be life-threatening. The moment of T1DM diagnosis in children has a psychoemotional impact on the patient, parent and entourage that needs to be managed psychologically, as well as the short- and long-term medical management of the disease itself. It represents a time when the lifestyle of the patient and entourage changes, and the monitoring for clinical signs of severity and for the occurrence of hyper-/hypoglycemia or metabolic, neurological, ophthalmological complications is essential. The patient with T1DM modifies his lifestyle, and adapts himself and those around him to the new condition: insulin deficiency.
As research advances, the autoimmune pathogenic hypothesis is supported by gradually deciphered mechanisms that explain the natural history of the disease. The 21st century offers explanations of pancreatic beta-cell destruction, with hopes and pathogenic links that may be therapeutic targets in the future.
Insulin dependence is lifelong, and today’s computerization offers the present option of automatic blood glucose monitoring and control via insulin pumps, which, especially in children, enhance the quality of life and make social integration easier.
Epidemiology
The prevalence of T1DM worldwide ranges from 0.8 to 4.6/1000 population, being 1-1.5/1000 in most countries. Differences in incidence have been reported by country, race, diagnosis or season of birth, age and gender.
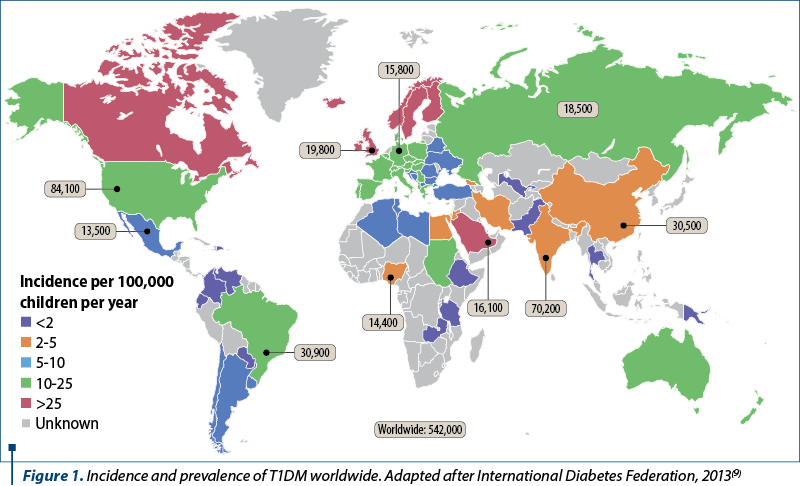
In 2013, the International Diabetes Federation published the sixth edition of its atlas (International Diabetes Federation, 2013)(9) which includes data from 219 countries. The incidence of T1DMD in children under the age of 14 years old ranges from 0.1/100,000 population per year in Papua New Guinea and Venezuela to 57.6/100,000 population in Finland. The results provided show a 576-fold variation between the populations analyzed worldwide. By race, non-Hispanic Caucasian subjects are the racial group with the highest incidence of T1DM, followed by Afro-Americans, Hispanic Caucasians and Asians. The highest incidence of T1DM is seen in the colder months(23).
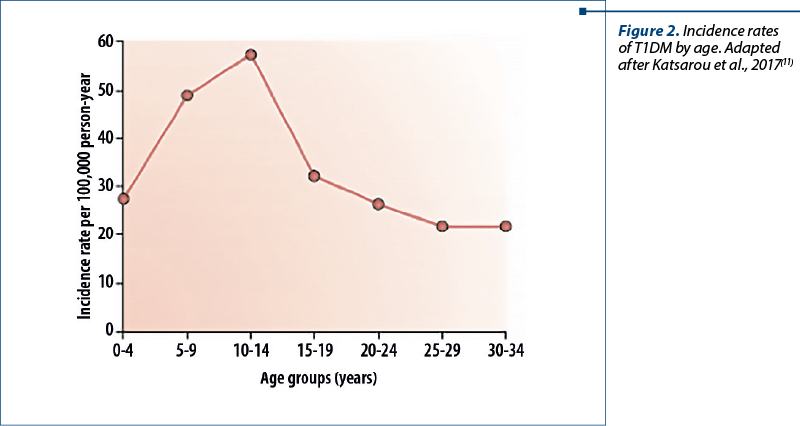
Incidence also varies by age group and gender. It is higher in childhood compared to adulthood. The highest incidence is found in the age group of 10-14 years old, and values decrease thereafter until the last decades of life(6). In people over 15 years old, a higher incidence is observed in males, while results in younger people are different in countries with high or low incidence of T1DM(17).
On the other hand, according to data reported by the EURODIAB group(20), its incidence in Europe has increased in recent decades, with an average annual increase of about 3.5%. In northern European countries, this increase has stopped in recent years(13).
Also striking are the differences in incidence between mainland Italy (8.4 cases per 100,000 inhabitants) and the island of Sardinia (36.9 cases per 100,000 inhabitants). These variations strongly support the importance of environmental factors in the development of type 1 diabetes. Most countries report that incidence rates have at least doubled in the last 20 years. The incidence appears to increase with the distance from the Equator(22).
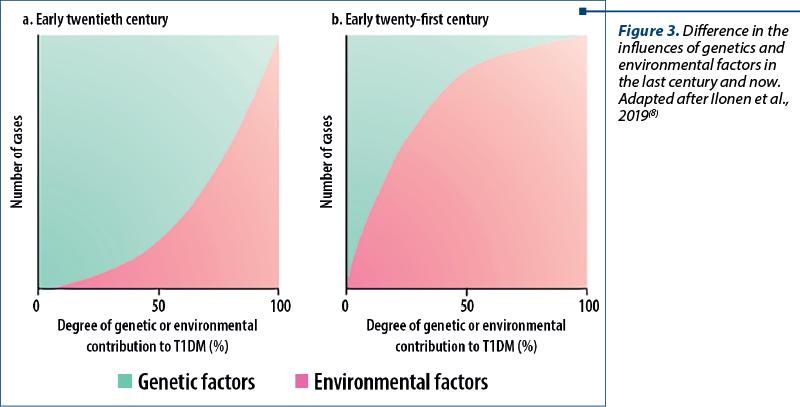
The variations described above may reflect a different degree of genetic susceptibility to diabetes or may be due to different exposure to environmental risk factors. It is known that T1DM is associated with the major histocompatibility complex HLA class II genes DR4 and DR3, and that the highest genetic susceptibility corresponds to the combination of DR3 and DR4 alleles. In an attempt to identify environmental triggers, the influence of socioeconomic level (the higher the level, the higher the incidence), association with viruses (especially enteroviruses), various dietary components (cow’s milk albumin, vitamin D, breastfeeding, wheat gluten, vitamin E), gut microbiome composition and maternal factors during pregnancy were analyzed. These analyses did not provide conclusive results regarding the etiopathogenesis of T1DM, nor did they allow its prevention(23).
Demographics by race
Different environmental effects on the development of T1DM complicate the racial influence, but racial differences are evident. Caucasian individuals have the highest reported incidence, while Chinese people have the lowest incidence. Type 1 diabetes is 1.5 times more likely to develop in Caucasian Americans than in Afro-Americans or Hispanics. Current evidence suggests that when immigrants from a low-incidence area move to a higher-incidence area, the rates of T1DM tend to rise toward the higher level.
Gender demographics
The influence of gender varies with overall incidence rates. Men are more at risk in high-incidence regions, especially older men, whose incidence rates often vary seasonally. Women appear to be more at risk in low incidence regions.
Age demographics
Type 1 diabetes can occur at any age, but the incidence rates generally increase with age until mid-puberty and then decline(3). The onset in the first year of life, although unusual, can occur, so T1DM should be considered in any infant or young child, as these children have the highest risk of mortality if the diagnosis is delayed. Since diabetes is easily missed in an infant or preschooler, if in doubt, the incidental presence of glycosuria is suggestive of the possibility.
The symptoms of T1DM in infants and young children may include the following:
-
moniliform rash
-
unexplained malaise
-
poor weight gain or weight loss
-
increased thirst
-
vomiting and dehydration.
In areas with high prevalence rates, a bimodal variation in incidence has been reported with a definite peak in early childhood (i.e., ages of 4-6 years old) and a second, much higher peak in incidence during early puberty (i.e., ages of 10-14 years old)(5).
T1DM morbidity is caused by its acute and chronic complications. Public information and education campaigns, as well as diabetes education for already diagnosed patients are important in preventing acute complications. Chronic complications can only be managed if the factors influencing their occurrence and progression are known.
Microangiopathy is characteristic of T1DM. Both retinopathy and nephropathy have recently been shown to have two etiopathogenic mechanisms. Diabetic retinopathy has both a microvascular and a neurological component with retinal involvement(24). Diabetic nephropathy has a predominantly vascular mechanism, reflecting systemic atherosclerosis – causing a decrease in glomerular filtration rate without albuminuria – and another classical mechanism, glomerulosclerosis, with albuminuria and no impairment of glomerular filtration rate until advanced stages(10).
The prevalence of diabetic retinopathy in T1DM is approximately 50% at 10 years and 70% at 20 years after disease onset. The prevalence of diabetic nephropathy at 20 years after disease onset varies between 20% and 40%. Diabetic neuropathy is a heterogeneous group of changes causing various clinical manifestations, although its most common presentations are represented by peripheral sensorimotor polyneuropathy and autonomic neuropathy. The form of diagnosis is essential to adequately assess the prevalence rates indicated in the various studies (20-50% for both peripheral sensory-motor polyneuropathy and autonomic neuropathy).
In the case of diabetic tripathy (retinopathy, nephropathy, and diabetic neuropathy), it has been reported, with some differences between pathologies and authors, that among the factors related to its onset and progression there are: glycemic control, age at diagnosis, duration of diabetes, hypertension, smoking, dyslipidemia, and genetic constitution. Of the modifiable factors, we know from the DCCT/EDIC study that improved glycemic control decreases the occurrence and progression of microangiopathy. The term metabolic memory was coined for the persistence of this effect over time(16). As a control goal, keeping blood glucose as close to normal as possible, with HbA1c<7%, is recommended in most consensus. Furthermore, if HbA1c is maintained below 7.6%, proliferative diabetic retinopathy and persistent macroalbuminuria can possibly be prevented for 20 years(18).
Although macroangiopathy is not a feature of diabetes, macrovascular complications in T1DM occur early, are more diffuse, and they have a faster progression and higher mortality, especially in patients younger than 40 years old. Patients with T1DM have at least a 10-fold increase in cardiovascular disease compared to age-matched non-diabetic patients. In addition, no differences in cardiovascular risk are observed between men and women with T1DM. Factors associated with microangiopathy include nephropathy, hypertension, smoking, dyslipidemia, disease duration, poor glycemic control, inadequate diet, sedentary lifestyle, obesity, and insulin resistance(14).
People with T1DM have an increased risk of mortality (relative risk of 3.82; 95% confidence interval [CI]; 3.41-4.29). At the age of 20 years old, men and women with T1DM have a lower life expectancy with 12.9 (95% CI; 11.7-14.1) and 11.1 years (95% CI; 10.1-12.1), respectively, compared to young people of the same age without diabetes. Reports on whether or not cancer mortality is increased in patients with T1DM give conflicting results. Before the age of 50, acute complications are the most common cause of death. From the age of 50 onwards, chronic diseases predominate, especially cardiovascular diseases. Blood glucose control and nephropathy contribute significantly to this outcome. However, in the absence of kidney disease and with adequate theoretical control (HbA1c<7), all-cause mortality is still more than twice as high compared to the general population (hazard ratio [HR] 2.36; 95% CI; 1.97-2.83), and cardiovascular mortality is almost three times higher (HR 2.92; 95% CI; 2.07-4.13). And when HbA1c is ≥9.7%, the HRs are: 8.51; 95% CI; 7.24-10.1 and 10.46; 95% CI; 7.62-14.37, respectively. Thus, although the mortality rates of T1DM have declined in recent decades, particularly in patients younger than 15 years old compared to pre-1990, they are still high and, in addition to glycemic control, hypertension and dyslipidemia, there are other factors involved that warrant the need for epidemiological research(21).
Etiopathogenesis
Insulin is essential for processing carbohydrates, fats and proteins. Insulin lowers blood glucose levels by allowing glucose to enter muscle cells and stimulating the conversion of glucose to glycogen (glycogenesis) as a carbohydrate store. Insulin also inhibits the release of stored glucose from liver glycogen (glycogenolysis) and slows the breakdown of fat into triglycerides, free fatty acids and ketones. It also stimulates fat storage. In addition, insulin inhibits the breakdown of proteins and fats to produce glucose (gluconeogenesis) in the liver and kidneys.
Hyperglycemia (i.e., a random blood glucose concentration higher than 200 mg/dL or 11 mmol/L) results when insulin deficiency leads to uninhibited gluconeogenesis and prevents the utilization and storage of circulating glucose. The kidneys cannot reabsorb the excessive glucose load, causing glycosuria, osmotic diuresis, thirst and dehydration. Increased breakdown of fats and proteins leads to ketone production and weight loss. Without insulin, a child with T1DM has an increased risk of death from diabetic ketoacidosis.
Insulin inhibits glycogenolysis and glycogenolysis while stimulating glucose uptake. In non-diabetic people, insulin production by pancreatic islet cells is suppressed when blood glucose levels fall below 83 mg/dL (4.6 mmol/L). If insulin is injected into a child with diabetes who has not consumed adequate amounts of carbohydrates, blood glucose levels gradually fall.
The brain depends on glucose for fuel. As glucose levels fall below 65 mg/dL (3.2 mmol/L), counterregulatory hormones (e.g., glucagon, cortisol, epinephrine) are released, and symptoms of hypoglycemia occur. These symptoms include sweating, shakiness, confusion, behavioral changes, and eventually coma when blood glucose levels fall below 30-40 mg/dL.
The glucose level at which symptoms occur varies greatly from individual to individual (and from time to time in the same individual), depending in part on the duration of diabetes, the frequency of hypoglycemic episodes, the rate of blood glucose decline, and the overall control. Glucose is also the sole source of energy for erythrocytes and the adrenal medulla.
A study by Chan et al. (2016) indicated that, in pediatric patients with type 1 diabetes, the presence of hypoglycemia is a sign of decreased insulin sensitivity, while hyperglycemia in these patients, especially overnight, signals improved insulin sensitivity. In contrast, other researchers have found evidence that, in pediatric patients with type 2 diabetes, metabolic syndrome markers and hyperglycemia are associated with reduced insulin sensitivity. The patients’ age from the study ranged from 12 to 19 years old(2).
Etiology
Most cases (95%) of T1DM are the result of the influence of environmental factors in a genetically susceptible person. This interaction leads to the development of an autoimmune disease directed against insulin-producing cells in the pancreatic islets of Langerhans. These cells are progressively destroyed, with insulin deficiency usually occurring after 90% of the islet cells have been destroyed.
Genetics
Strong evidence suggests a genetic component in type 1 diabetes. Monozygotic twins have a 60% lifetime concordance for developing type 1 diabetes, although only 30% do so within 10 years of the first twin’s diagnosis. In contrast, dizygotic twins have a concordance risk of only 8%, which is similar to the risk among their other siblings.
The frequency of developing diabetes in children with a mother who has diabetes is 2-3%; this figure increases to 5-6% for children with a father who has type 1 diabetes. The risk for children increases to almost 30% if both parents are diabetic.
Human leukocyte antigen (HLA) class II molecules DR3 and DR4 are strongly associated with type 1 diabetes. More than 90% of Caucasians with type 1 diabetes express one or both molecules, compared with 50-60% of the general population.
Patients expressing DR3 are also at risk of developing other autoimmune endocrinopathies and celiac disease. These patients are more likely to develop diabetes at an older age, have positive islet cell antibodies, and appear to have a longer period of residual islet cell function.
Patients expressing DR4 are usually younger at diagnosis and more likely to have positive insulin antibodies, but they are unlikely to have other autoimmune endocrinopathies. The expression of both DR3 and DR4 carries the highest risk of type 1 diabetes; these patients have characteristics of both DR3 and DR4 groups.
Neonatal diabetes, including diagnosis in infants less than 6 months of age, is most likely due to an inherited defect in the Kir6.2 subunit potassium channel of islet beta cells, and genetic screening is indicated(7). This is particularly important, as these infants respond well to sulfonylurea therapy.
Environmental factors
The environmental factors are important, because even identical twins have only 30-60% concordance for type 1 diabetes, and because incidence rates vary in genetically similar populations under different living conditions(19). No single factor has been identified, but infections and diet are considered the two most likely environmental candidates.
Viral infections may be the most important environmental factor in the development of type 1 diabetes, probably by initiating or modifying an autoimmune process. Cases of direct toxic effect of infection in congenital rubella have been reported. One study suggests that enteroviral infection during pregnancy carries an increased risk of type 1 diabetes in offspring. Paradoxically, the incidence of type 1 diabetes is higher in areas where the overall burden of infectious diseases is lower.
A US CDC study indicates that the infection with SARS-CoV-2, the virus that causes COVID-19 disease, increases the likelihood of developing diabetes in children under the age of 18 more than 30 days after infection. The researchers, using two US medical claims databases, reported that pediatric patients with COVID-19 in the HealthVerity database were 31% more likely than other young people to be newly diagnosed with diabetes, while those in the IQVIA database were 166% more likely. The study could not specify the type or types of diabetes specifically linked to COVID-19, with the report stating that the disease could cause both type 1 and type 2 diabetes, but by different mechanisms. The researchers suggested, however, that COVID-19 may induce diabetes by directly attacking pancreatic cells expressing ACE2 receptors, that it may give rise to diabetes “through stress hyperglycemia resulting from cytokine storm and infection-induced changes in glucose metabolism”, or that COVID-19 may cause diabetes by converting prediabetes to diabetes. It was also not known whether the diabetes is transient or chronic(1,25).
A study by Kendall et al. (2022) found that, compared to pediatric subjects with a non-SARS-CoV-2 respiratory infection, the proportion of children who were diagnosed with new-onset type 1 diabetes within six months after a SARS-CoV-2 infection was 72% higher. According to the researchers, who looked at patients aged 18 years old or younger, the rate of new-onset type 1 diabetes in the two groups was 0.025% and 0.043%, respectively, at six months(12).
However, a study by Cromer et al. (2022) looked at adult patients with newly diagnosed diabetes at the time of hospital admission for COVID-19, finding that a number of them subsequently regressed to a state of normoglycemia or prediabetes. The investigators reported that, of the 64 survivors in the study with newly diagnosed diabetes (62 of whom had type 2 diabetes), 26 (40.6%) were known to have regressed (median follow-up: 323 days)(4).
Diet
Dietary factors are also relevant. Breastfed infants have a lower risk of type 1 diabetes, and a direct relationship is observed between per capita cow’s milk consumption and diabetes incidence. Some proteins in cow’s milk (e.g., bovine serum albumin) have antigenic similarities to an islet cell antigen.
Nitrosamines – chemicals found in smoked foods and some water sources – are known to cause type 1 diabetes in animal models; however, no clear link has been made to humans.
The known association of increased incidence of type 1 diabetes with distance from the Equator may now have an explanation. Reduced exposure to ultraviolet (UV) light and lower vitamin D levels – both of which are more likely to be found at higher latitudes – are associated with an increased risk of type 1 diabetes(15).
Chemical causes
Streptozotocin and RH-787, a rat poison, selectively damage islet cells, and they can cause type 1 diabetes.
Other causes
Additional factors in the development of T1DM include the following:
-
congenital absence of pancreas or of islet cells
-
pancreatectomy
-
pancreatic disease (e.g., cystic fibrosis, chronic pancreatitis, thalassemia major, hemochromatosis, hemolytic uremic syndrome)
-
Wolfram syndrome (diabetes insipidus, diabetes mellitus, optic atrophy, deafness [DIDMOAD])
-
chromosomal disorders, such as Down syndrome, Turner syndrome, Klinefelter syndrome or Prader-Willi syndrome (the risk is said to be about 1% for Down and Turner syndromes).
Natural history of T1DM
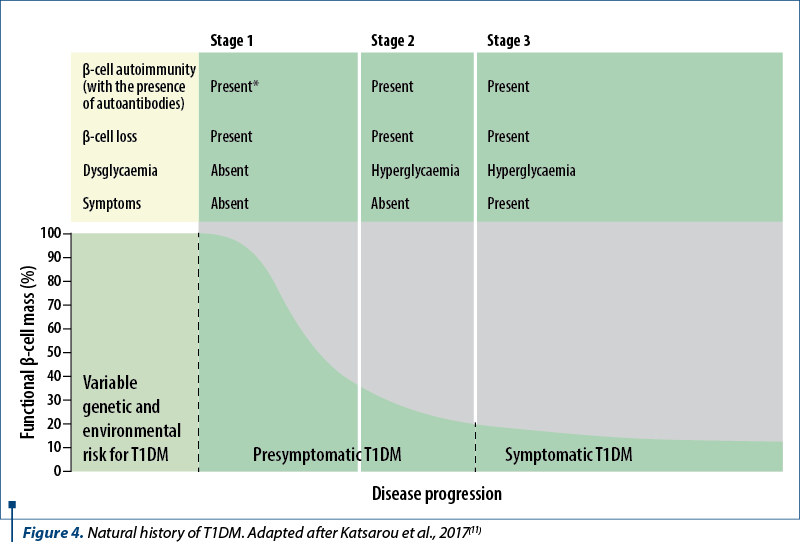
The development of T1DM occurs in three stages:
-
Stage 1 is asymptomatic and is characterized by normal fasting blood glucose, normal glucose tolerance and the presence of ≥2 pancreatic autoantibodies.
-
The diagnostic criteria in stage 2 include the presence of pancreatic autoantibodies (usually multiple) and dysglycemia – fasting blood glucose (fasting blood glucose between 100 and 125 mg/dL) or impaired glucose tolerance (blood glucose at 2 hours after 75 g of glucose between 140 and 199 mg/dL) or an HbA1c between 5.7% and 6.4%. The individuals remain asymptomatic.
-
In stage 3, there is diabetes, defined by hyperglycemia (random glucose ≥200 mg/dL) with clinical symptoms, fasting glucose ≥126 mg/dL, glucose ≥200 mg/dL two hours after the ingestion of 75 g of glucose during an oral glucose tolerance test, and/or HbA1c≥6.5%. If the individual does not have classic symptoms of hyperglycemia or hyperglycemic crisis, two glucose tolerance tests (simultaneously or at different times) are recommended to confirm the diagnosis. If there is an acute onset of symptoms with hyperglycemia, as is more common in young-onset T1DMD, HbA1c may be misleading at the time of diagnosis, and glucose tolerance testing criteria should be used.
Conclusions
Type 1 diabetes mellitus in children, although not impressive in epidemiological terms, is a chronic autoimmune disease with long-term adverse consequences if inadequately controlled. The autoimmune pathogenesis hypothesis, through gradually deciphered mechanisms that explain the natural history of the disease, offers explanations of pancreatic beta cell destruction, with hopes and pathogenic links that may be therapeutic targets in the future. With good care, sufferers have the prospect of living normal, healthy lives.
Funding: No funding to declare.
Corresponding author: Bogdan A. Stana E-mail: bogdan.stana@gmail.com
Conflict of interest: none declared.
Financial support: none declared.
This work is permanently accessible online free of charge and published under the CC-BY licence.

Bibliografie
2. Chan CL, Pyle L, Morehead R, Baumgartner A, Cree-Green M, Nadeau KJ. The role of glycemia in insulin resistance in youth with type 1 and type 2 diabetes. Pediatr Diabetes. 2017;18(6):470-477.
3. Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009;10 Suppl 12:3-12.
4. Cromer SJ, Colling C, Schatoff D, et al. Newly diagnosed diabetes vs. preexisting diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Complications. 2022;36(4):108145.
5. Felner EI, Klitz W, Ham M, Lazaro AM, Stastny P, Dupont B. Genetic interaction among three genomic regions creates distinct contributions to early- and lateonset type 1 diabetes mellitus. Pediatr Diabetes. 2005;6(4):213-20.
6. Forga L, Goñi MJ, Cambra K, et al. Diferencias por edad y sexo en la incidencia de diabetes tipo 1 en Navarra (2009-2011) [Differences by age and gender in the incidence of type 1 diabetes in Navarre, Spain (2009-2011)]. Gac Sanit. 2013;27(6):537-540.
7. Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350(18):1838-49.
8. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15(11):635-650.
9. International Diabetes Federation. IDF Diabetes Atlas, 6th Edition. International Diabetes Federation, 2013.
10. Jindal A, Garcia-Touza M, Jindal N, Whaley-Connell A, Sowers JR. Diabetic kidney disease and the cardiorenal syndrome: old disease, new perspectives. Endocrinol Metab Clin North Am. 2013;42(4):789-808.
11. Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016.
12. Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 Infection with New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021. JAMA Netw Open. 2022;5(9):e2233014.
13. Lund-Blix NA, Stene LC, Rasmussen T, Torjesen PA, Andersen LF, Rønningen KS. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: the MIDIA Study. Diabetes Care. 2015;38(2):257- 263.
14. Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):625-640.
15. Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51(8):1391-8.
16. Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16.
17. Negrato CA, Dias JP, Teixeira MF, et al. Temporal trends in incidence of Type 1 diabetes between 1986 and 2006 in Brazil. J Endocrinol Invest. 2010;33(6):373-377.
18. Nordwall M, Abrahamsson M, Dhir M, Fredrikson M, Ludvigsson J, Arnqvist HJ. Impact of HbA1c, followed from onset of type 1 diabetes, on the development of severe retinopathy and nephropathy: the VISS Study (Vascular Diabetic Complications in Southeast Sweden). Diabetes Care. 2015;38(2):308-315.
19. Patterson CC, Carson DJ, Hadden DR. Epidemiology of childhood IDDM in Northern Ireland 1989-1994: low incidence in areas with highest population density and most household crowding. Northern Ireland Diabetes Study Group. Diabetologia. 1996;39(9):1063-9.
20. Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142-2147.
21. Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313(1):37-44.
22. Soltesz G, Patterson CC, Dahlquist G; EURODIAB Study Group. Worldwide childhood type 1 diabetes incidence – what can we learn from epidemiology?. Pediatr Diabetes. 2007;8 Suppl 6:6-14.
23. Stanescu DE, Lord K, Lipman TH. The epidemiology of type 1 diabetes in children. Endocrinol Metab Clin North Am. 2012;41(4):679-694.
24. Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci (Lond). 2013;125(1):1-17.
25. Tucker ME. COVID-19 Associated with Increased Diabetes Risk in Youth. Medscape Medical News. 2022 Jan 10. https://www.medscape.com/viewarticle/966291
Articole din ediţiile anterioare
Aspiraţia de corp străin - prezentare de caz
Aspirația de corp străin la copilul mic reprezintă o urgență medicală. Evoluția depinde de natura și de volumul corpului străin, de posibilitat...
Variation of pNGAL values in a batch of pediatric patients with chronic kidney disease from northeastern Romania
Incidenţa insuficienţei renale cronice (IRC) şi mortalitatea prin IRC la copii şi adolescenţi sunt în creştere.
Apendicectomia laparoscopică versus cea deschisă în tratamentul apendicitei acute la copii
Appendectomy has been the standard of care for appendicitis since the late 1800s.
Modern treatment methods to reduce mortality and morbidity associated with burns in the pediatric patient
Arsurile pot avea diverse etiologii şi diferite suprafeţe şi grade. În funcţie de gravitate, se pot însoţi de morbiditate şi mortalitate sem...