Burns can have different etiologies and different areas and degrees. Depending on the severity, they can be accompanied by significant morbidity and mortality. Prompt diagnosis and appropriate treatment decrease the duration of healing and chronic complications. Alongside skin grafts, there are a multitude of modern treatment techniques, such as negative pressure therapy or skin substitutes, that decrease the inflammatory response and the infectious risk, and promote healing. The use of these methods depends on the doctors’ experience, product availability and cost-benefit ratio.
Modern treatment methods to reduce mortality and morbidity associated with burns in the pediatric patient
Metode moderne de tratament pentru scăderea mortalităţii şi morbidităţii pacientului pediatric cu arsuri
First published: 30 iunie 2023
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.70.2.2023.8302
Abstract
Rezumat
Arsurile pot avea diverse etiologii şi diferite suprafeţe şi grade. În funcţie de gravitate, se pot însoţi de morbiditate şi mortalitate semnificative. Diagnosticul rapid şi tratamentul adecvat reduc durata de vindecare şi complicaţiile cronice. Alături de grefele de piele, există o multitudine de tehnici moderne de tratament, cum ar fi terapia cu presiune negativă sau substituenţii cutanaţi, care scad răspunsul inflamator şi riscul infecţios şi promovează cicatrizarea. Folosirea acestor metode depinde de experienţa medicilor, de disponibilitatea produselor şi de raportul cost-beneficiu.
Introduction
Burns cause severe complications that can have serious social, psychological and potentially disabling repercussions for patients. The treatment of burn injuries has received more attention over the years, leading to the development of artificial skin or various medical devices to facilitate the care of burn patients and to prevent some of the acute or chronic phase complications such as post-traumatic stress, vicious scarring or malignant skin tumors on burn scars.
Skin substitutes are used to treat burn injuries. Various biomaterials are available to produce skin substitutes, including natural biopolymers and synthetic polymers. A composite matrix containing both natural and synthetic biomaterials can thus form a substitute that meets all the clinical requirements, matching the size and type of the wound, the degree of burn and the age of the patient.
Treatment methods for burns
In addition to the classic methods of debridement, chemical toileting and the use of skin grafts, there are modern methods that can decisively influence the course of a pediatric burn patient.
Negative pressure therapy
Negative pressure therapy systems are a noninvasive method of treatment that involves exposing the injured area to controlled sub-atmospheric pressures via a suction pump. It was first applied in 1987 to treat infected wounds and soft tissue trauma. This method is indicated in the treatment of wounds that heal very slowly, such as traumatic, postoperative, pre- and post-skin transplant wounds. The application of negative pressure and special dressing kits removes excess fluid from the wound and improves blood flow, which will help the wound to heal.
Negative pressure wound therapy without instillation is intended to create an environment that contributes to wound healing by secondary or tertiary (delayed primary) intention, preparing the wound bed for closure, reducing edema, stimulating granular tissue formation and perfusion, and removing exudate and infectious material. The instillation option is indicated for patients who can benefit from vacuum-assisted drainage and controlled administration of solutions and suspensions for topical wound treatment at the wound bed(1-4).
The negative pressure wound therapy system, with and without instillation, is indicated for patients with chronic, acute, traumatic, subacute and dehiscent wounds, second-degree burns, ulcerations (such as diabetic foot, scars or venous ulcers), flaps and grafts(5).
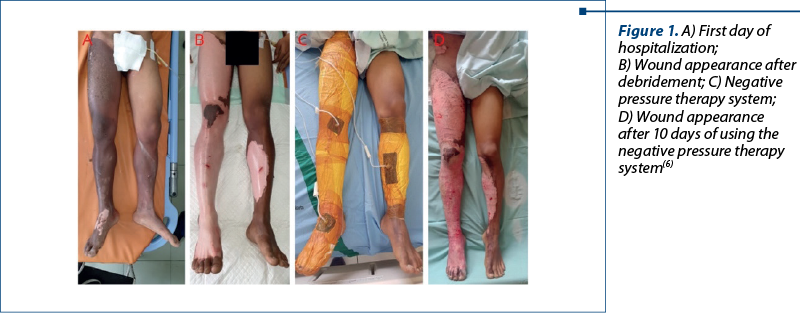
The effectiveness of this method was demonstrated in a study by Dr. Lutfi Kirdar, in which negative pressure wound therapy was applied using a system including wound-covering sponges, connectors between the wound and the device, collector and negative pressure device (Figure 1)(6).
In all patients, escharotomy and wound debridement were performed in the operating room, and dressings were changed every 72 hours. Thirty-eight patients were evaluated in this study, three of whom did not survive due to the severity of their injuries. No complications were reported during the study, no outbreaks of bacteria, and the average time to wound closure was 11 days. The reduction of edema and the appearance of granulation were observed in all patients. This study demonstrated that negative pressure therapy can reduce the number of wound debridement procedures, reduce wound closure time and length of hospitalization(6).
Biomaterials and skin substitutes
Patients with extensive burns require the use of skin substitutes due to the lack of sufficient donor tissue. Various biomaterials are available to produce skin substitutes, including natural biopolymers and synthetic polymers. Natural biopolymers offer improved cellular response, while synthetic polymers offer better control over chemical composition and mechanical properties. A composite matrix containing both naturally occurring and synthetic biomaterials can form a substitute that meets all the clinical requirements, matching the size and type of wound, the degree of burn and the age of the patient.
Skin grafts are considered the standard for treating burn wounds, but complications associated with harvesting them have led to the need to develop skin substitutes. These should meet the structure of the dermis and epidermis and fulfil their properties and functional requirements.
Collagen is the main component of the extracellular matrix and is widely used for various biomedical applications, including wound healing and skin regeneration. The collagen matrix in the dermis acts to cushion mechanical shocks and provides strength. It can be extracted from any animal tissue and its properties depend on the origin of the extraction tissue, such as bovine skin, tendons, pig skin, etc. However, animal collagen carries the risk of pathogen transmission. Collagen matrices are mainly made by freeze-drying, electrospinning and bioprinting. It is the most widely used biomaterial in the formation of skin substitutes such as Integra®, OrCel®, Promogran®, etc.
Gelatin is a denatured form of collagen that is obtained by controlled hydrolysis. It is structurally composed of the triple amino acids glycine, proline and hydroxyproline. It enhances cell-matrix interactions due to integrin receptors in cell membranes and positive charges from the amino acids lysine and arginine that facilitate the attachment of negatively charged cell membranes. Gelatin is used for dermal and epidermal regeneration by inserting cells such as fibroblasts, keratinocytes and hair follicle stem cells, resulting in efficient skin regeneration and good reepithelization, promoting vascularization of the treated area. This biomaterial has a biocompatibility similar to that of collagen but is more economically feasible due to its high solubility and low cost.
Elastin makes up 2-4% of the skin and elastin fibers are responsible for skin elasticity. In vivo, it occurs as water-soluble tropoelastin monomers that bind to insoluble elastin. The use of elastin for a skin substitute improves wound healing and reepithelization. Tropoelastin is the main precursor used in the production of electrospun skin substitutes. Used as an additive in the matrices from which skin substitutes are made, it enhances cell proliferation and wound healing.
Fibrin hydrogels are formed by the polymerization of fibrinogen activated by thrombin protease. After damage to blood vessels, a fibrous network composed of fibrin is formed during hemostasis, providing a temporary matrix for the attachment and migration of cells involved in early-stage wound healing. Due to its matrix vascularization potential, fibrin is used in the production of skin substitutes.
Chitosan is a polysaccharide derived from chitin, the amino groups in chitosan provide positive charges, so it is the only naturally occurring positively charged polysaccharide. This phenomenon allows chitosan to interact with negatively charged species such as red blood cells. It is an antibacterial, antifungal, mucoadhesive, analgesic and hemostatic biomaterial that does not trigger inflammation after implantation. It is used in combination with other materials such as collagen, gelatin and fibrin to make dressings or skin substitutes in the form of sponges or hydrogels.
Synthetic polymers have the advantage that their composition and reproducibility can be controlled. Polylactic acid is an aliphatic, biodegradable polyester that has been shown to promote vascularization when incorporated with other biomaterials. Polyethylene glycol is used as a dermal substitute by adding dermal fibroblasts, collagen, conferring cell adhesion, short-term degradation and minimal inflammation(7).
Cellulose and chitosan-based biopolymer hydrogels have demonstrated excellent wound healing characteristics. Hydrogels cross-linked with synthetic polymers have mechanical properties that provide good elasticity, flexibility, compressive stress, Young’s modulus and tensile stresses. Biomaterial dressings have excellent antibacterial efficacy against Gram-negative and Gram-positive strains of bacteria that lead to wound infection. These properties enhance rapid reepithelization, granulation tissue development and wound healing(8).
Nanotechnology and medicine offer major opportunities to treat wounds of burn patients. Nanoparticles are beneficial to improve the therapeutic efficacy of synthetic compounds, they can act on cells in the healing process. New therapeutic approaches are needed to prevent the incidence of infection and sepsis, and nanomedicine is a promising area as it improves quality of life at a low cost.
Ideal nanoparticles must meet a number of conditions, including immunogenicity, safety and efficiency. Metal nanoparticles have been used for various purposes such as medical imaging, diagnostics, drug delivery or antimicrobial coatings. Their use in treating infections is indicated because they possess antimicrobial properties. Silver nanoparticles have antimicrobial activity due to their ability to disrupt the plasma membrane, which alters permeability, leading to lysis of the microorganism. The choice of nanoparticles is influenced by the choice of drug type and the severity of the lesion. Dressings consisting of sponges, films, membranes or hydrogels provide protection against microorganisms and accelerate the healing process. Future prospects in burn treatment include the use of mesenchymal stem cells and progenitor cells in combination with nanomedicines, as these cells play a role in skin regeneration(9).
Role of pluripotent stem cells
The production of artificial tissues is a fundamental part of tissue treatment for burn patients. The essential components of human tissue engineering include cells and biomaterials so that porous three-dimensional matrices function as matrices for tissue regeneration. Stem cells are among the most important components of tissue engineering and have the property of replication, being able to produce multiple stem cells from a single cell, differentiating into different cell types.
Embryonic stem cells were first introduced in the early 1980s directly from rat blastocysts and are capable of differentiating into three cell lines. Adult stem cells have been designated for differentiation into specific pathways, including maintenance, production and replacement of fully differentiated cells(10).
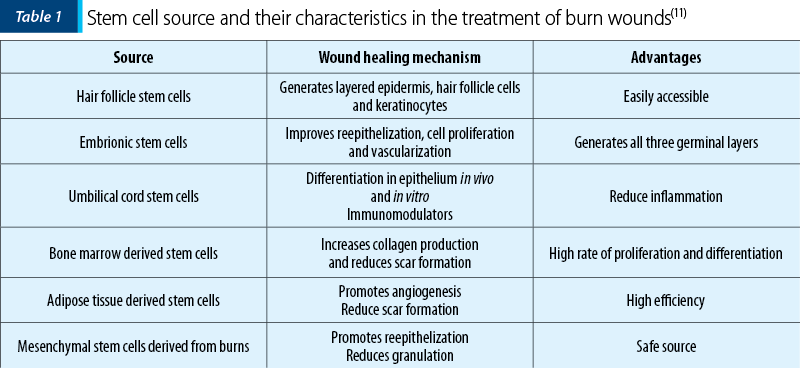
Stem cells are used for burn wound care because they have the ability to secrete growth factors, respond to local stimuli, and influence the wound microenvironment to promote wound healing(11-15).
Three-dimensional bioprinting is one of the most modern techniques used in tissue engineering that offers the possibility of integrating multiple cell types, including stem cells, into organized and functional tissue structures, such as skin, in a matrix. Technically, 3D bioprinting can mimic not only the layered structure and complex composition of natural skin, but also the construction of skin appendages such as hair follicles, sweat glands, sebaceous glands needed in clinical application(16).
There are a number of challenges to the use of stem cells on burn wounds, the predominant one being their effective application to the burn wound. They can be administered locally or intravenously and their localization after transplantation is followed in vivo by genetic imaging and PET scans.
Stem cells therefore accelerate wound healing through a complex series of pathways that aim to promote neo-angiogenesis, collagen deposition and granulation tissue formation.
Devices used to treat burns
Surgical interventions for burn patients require autografts which involve surgical excision and transplantation to the site of the lesion, and these are the current standard of care for treating severe burns. However, autografting carries the risk of various donor complications such as pain, scarring and infection. In pediatric patients, autograft is not the best solution because of the reduced donor surface area.
Technological advances in the development of skin substitutes have provided alternatives for burn care, developing a wide range of products that can reduce or eliminate the need for autograft in patients with severe burns.
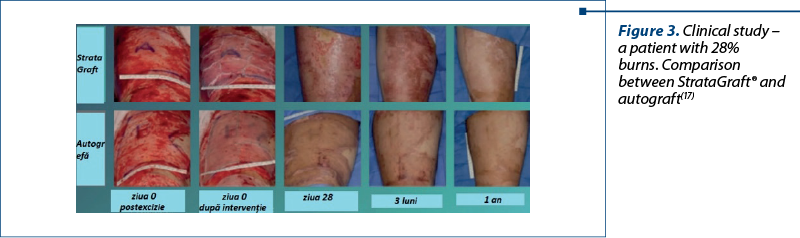
StrataGraft® (allogeneic cultured keratinocytes and dermal fibroblasts in collagen) is a bioengineered allogeneic cellularized composition that was approved in the United States of America in 2021 for the treatment of deep partial-thickness thermal burns. The fully layered epidermis contains metabolically active cells that produce and secrete a variety of growth factors and cytokines(13). It is contraindicated in patients with known allergies to murine collagen or products containing bovine or porcine ingredients.
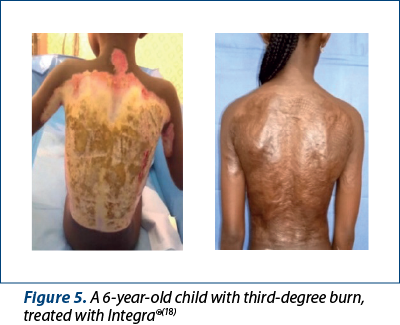
Integra® is the most widely accepted synthetic skin substitute and is widely used in burn patients. It is composed of a bilaminated membrane, consisting of a bovine collagen-based dermal analogue of glycosaminoglycans and chondroitin-6-sulphate, and a temporary epidermal silicone substitute layer. It was originally created out of the need to provide temporary coverage to patients with extensive burns. However, its clinical applications have expanded significantly as Integra® serves as a matrix for neodermis growth. Blood vessels and other cells migrate into the matrix and begin to deposit a new layer of dermis. The impermeable silicone layer serves to close the wound and prevent fluid loss.
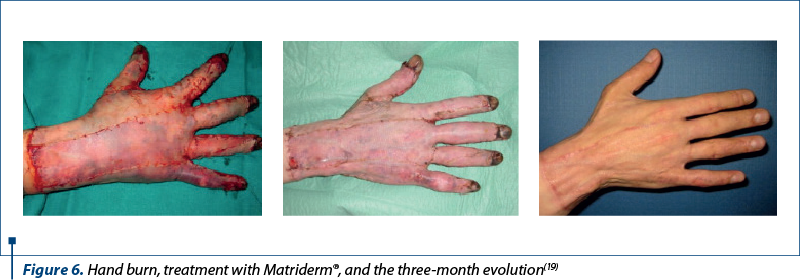
Matriderm® is a unique collagen and elastin matrix that replaces the skin and is able to closely resemble the human dermis, accelerating cell invasion, cell elongation and proliferation, limiting myofibroblast formation and contraction.
Collagen is obtained from bovine dermis and contains dermal collagen types I, III and V. Elastin is obtained from bovine nuchal ligament by hydrolysis. It modulates the formation of scar tissue and has excellent hemostatic properties, reducing the risk of subgrafted hematoma with split skin. It is supplied in sterile packaging and can only be used under sterile conditions as well. Requiring rehydration in saline, the matrix is ready for use as soon as the appearance of the entire surface has changed from white to translucent(19).
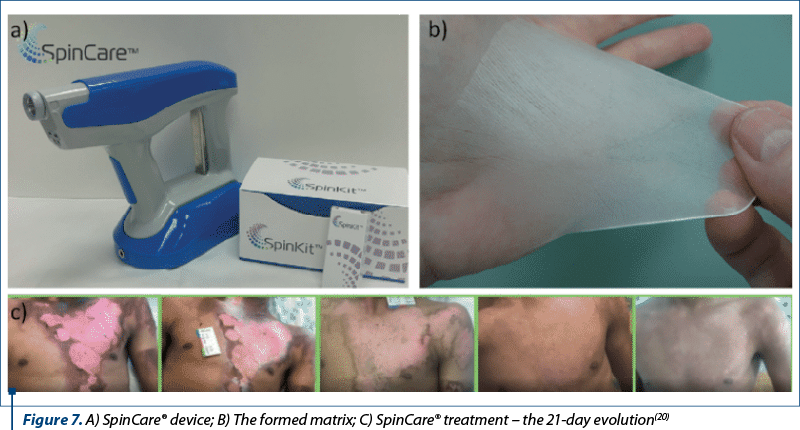
SpinCare Nanomedic® is the first and only fully customized, portable electrospinning wound care device that addresses post-burn wound care. The system imprints a matrix directly onto the patient, which acts as a temporary skin substitute and provides the wound with an optimal environment for healing. The porous structure allows drainage while acting as a barrier against microorganisms, reducing the risk of infection. The whole treatment can take 30 seconds or one minute, depending on the size of the area to be covered(20).
RenovaCare® is an experimental burn treatment option invented in 2008 that works like a paint gun to spray your own skin cells onto a burn. The skin cells are mixed with enzymes, and the sample is then mixed with a buffer solution. The final step is to filter the cells and create a liquid, called a regenerative epithelial suspension, which contains all the skin cell types needed for optimal healing. As an alternative to nonoperative management, RenovaCare® can achieve rapid wound re-epithelialization, especially of large wounds(21).
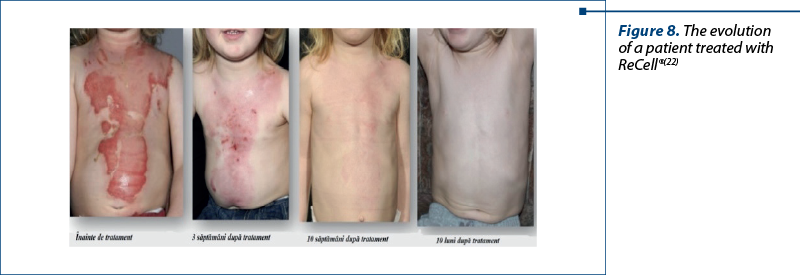
ReCell® uses a unique combination of enzymes to break down layers of skin in a piece of tissue, then mixes them into a liquid that can be applied to the skin using a low-tech spray syringe. The ReCell® autologous cell harvesting device was developed to minimize the amount of healthy skin that needs to be transplanted to treat burn wounds. This technique offers a unique epidermal regeneration strategy aimed at the definitive closure after excision of a burn. The treatment represents a new strategy for the treatment of burns and aims to provide alternatives for autograft(22,23).
Conclusions
Morbidity and mortality are essential indicators for assessing the health status of a population, identifying health needs and developing policies and interventions for disease prevention and management. Reducing morbidity and mortality is an important objective of health systems, and this can be achieved by promoting preventive measures, early diagnosis, appropriate treatment and improved access to quality healthcare.
In recent years, remarkable technological advances have opened new horizons for improving the quality of life and patients outcomes, bringing advanced tools, innovative methods and promising applications.
The production of artificial tissues is a fundamental part of tissue treatment for burn patients. The essential components of human tissue engineering include cells and biomaterials so that porous three-dimensional matrices function as matrices for tissue regeneration. Stem cells are among the most important components of tissue engineering and have the property of replication, being able to produce multiple stem cells from a single cell, differentiating into different cell types.
The development of new technologies that produce skin substitutes using stem cells, polymers or nanoparticles can have a decisive impact on the treatment of a burn patient.
Conflict of interest: none declared
Financial support: none declared
This work is permanently accessible online free of charge and published under the CC-BY.



d
Bibliografie
- Hoeller M, Schintler MV, Pfurtscheller K, Kamolz LP, Tripolt N, Trop M. A retrospective analysis of securing autologous split-thickness skin grafts with negative pressure wound therapy in paediatric burn patients. Burns. 2014;40(6):1116-1120.
- Kantak NA, Mistry R, Halvorson EG. A review of negative-pressure wound therapy in the management of burn wounds. Burns. 2016;42(8):1623-1633.
- Resadita R, Seswandhana MR, Purnomo E, et al. The effect of NPWT in wound healing and bacterial count on deep dermal burn injury model: An experimental study. Ann Med Surg (Lond). 2022;75:103367.
- Chu H, Maklad M, Cobley T, Sen S. The use of negative pressure wound therapy (NPWT), as an adjunct for primary wound healing, in deep partial-thickness paediatric foot burns: A case report. Burns Open. 2020;4(2):60-63.
- Sistem de terapie cu presiune negativă la nivelul plăgilor V.A.C.ULTA™ (sistem de terapie v.a.c.ulta™) informaţii despre siguranţă, Manual de utilizare, Acelity, 2018.
- Kement M, Başkıran A. Efficacy of negative pressure wound therapy in the management of acute burns. Turkish Journal of Trauma and Emergency Surgery. 2018;24(5):412-416.
- Rosadi Seswandhana M, Anzhari S, Dachlan I, Widodo Wirohadidjojo Y, Aryandono T. A case series of negative pressure wound therapy as a promising treatment in patients with burn injury. Int J Surg Case Rep. 2020;69:64-67.
- Sheikholeslam M, Wright MEE, Jeschke MG, Amini-Nik S. Biomaterials for Skin Substitutes. Adv Healthc Mater. 2018;7(5):10.1002/adhm.201700897.
- Alven S, Aderibigbe BA. Chitosan and cellulose-based hydrogels for wound management. International Journal of Molecular Sciences. 2020;21(24):9656.
- Souto EB, Ribeiro AF, Ferreira MI, et al. New Nanotechnologies for the Treatment and Repair of Skin Burns Infections. Int J Mol Sci. 2020;21(2):393.
- Lotfalah Moradi S, Mahmoodinia Maymand M, Ardeshirylajimi A. Application of induced pluripotent stem cells in tissue engineering. Advances in Stem Cell Biology. 2022;17:483-505.
- Watt SM, Pleat JM. Stem cells, niches and scaffolds: applications to burns and wound care. Advanced Drug Delivery Reviews. 2018;123:82-106.
- Ullah S, Mansoor S, Ayub A, et al. An update on stem cells applications in burn wound healing. Tissue Cell. 2021;72:101527.
- Ozturk S, Karagoz H. Experimental stem cell therapies on burn wound: do source, dose, timing and method matter?. Burns. 2015;41(6):1133-1139.
- Abouzaid AM, El Mokadem ME, Aboubakr AK, Kassem MA, Al Shora AK, Solaiman A. Effect of autologous fat transfer in acute burn wound management: A randomized controlled study. Burns. 2022;48(6):1368-1385.
- Abdul Kareem N, Aijaz A, Jeschke MG. Stem Cell Therapy for Burns: Story so Far. Biologics. 2021;15:379-397.
- Thomas DJ, Jessop ZM, Whitaker IS. 3D Bioprinting for reconstructive surgery. Elsevier, 2018, 367-379.
- Holmes Iv JH, Cancio LC, Carter JE, Faucher LD, Foster K, Hahn HD, King BT, Rutan R, Smiell JM, Wu R, Gibson ALF. Pooled safety analysis of STRATA2011 and STRATA2016 clinical trials evaluating the use of StrataGraft® in patients with deep partial-thickness thermal burns. Burns. 2022;48(8):1816-1824.
- Nguyen DQ, Potokar TS, Price P. An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns. 2020;36(1):23-8.
- https://matriderm.com/en, accesat decembrie 2022.
- https://nanomedic.com/spincare, accesat decembrie 2022.
- Esteban-Vives R, Corcos A, Choi MS, et al. Cell-spray auto-grafting technology for deep partial-thickness burns: Problems and solutions during clinical implementation. Burns. 2018;44(3):549-559.
- Holmes JH 4th, Molnar JA, Shupp JW, et al. Demonstration of the safety and effectiveness of the RECELL® System combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns. 2019;45(4):772-782.
Articole din ediţiile anterioare
Actualităţi în etiopatogenia diabetului zaharat de tip 1 la copil
Diabetul zaharat de tip 1 (DZ tip 1) reprezintă o patologie autoimună impresionantă prin potenţialul complicaţiilor pe care le poate induce de-a lu...
Aspecte fiziopatologice în tulburarea hiperactivitate/deficit de atenţie
Tulburarea hiperactivitate/deficit de atenție (ADHD) este cea mai frecventă afecțiune psihiatrică la copiii de vârstă școlară, a cărei prevalență e...
Secvenţa ABC sau secvenţa CAB în resuscitarea cardiopulmonară pediatrică?
O resuscitare cardiopulmonară de calitate este esenţială în cazul unui pacient aflat în stop cardiac. Şansele de supravieţuire şi calitatea ulteri...
Malnutriţia acută severă, consecinţă a statusului socioeconomic precar – serie de cazuri clinice
Malnutriţia severă la copil constituie o problemă importantă la nivel mondial. Deficitele protein-calorice şi ale micronutrienţilor pot cuprinde ma...