Introduction. Pediatric acute liver failure (ALF) is a rare but severe condition characterized by coagulopathy and laboratory findings of acute liver injury in a patient without any history of previous hepatic disease. The infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is often associated with digestive symptoms in children, but the liver involvement is usually mild and self-limited. Case report. We report the case of a 5-year-old previously healthy boy who presented sudden onset of jaundice, associated with pale stools, dark urine, abdominal pain and vomiting. The laboratory findings revealed severe coagulopathy (prothrombin time: 126.9 s, international normalized ratio: 6.14), hypoglycemia, hypoalbuminemia, increased transaminases (aspartate aminotransferase: 1254 IU/L, alanine aminotransferase: 1548 IU/L), direct hyperbilirubinemia (total bilirubin: 27.69 mg/dL, direct bilirubin: 20.74 mg/dL). An extensive etiological evaluation was performed and the common infectious, metabolic, autoimmune and toxic causes of pediatric ALF were excluded. Wilson’s disease was initially suspected, based on the low levels of ceruloplasmin and the high 24-hour urinary copper excretion, but the values normalized on following determinations. Abdominal ultrasound showed hepatomegaly, increased liver echogenicity, gallbladder wall thickening with parietal microabscesses in the absence of gallstones, and mild ascites. Real-time PCR for SARS-CoV-2 was negative on admission, but serology revealed positive IgG antibodies in high titer with negative IgM antibodies. The patient was managed conservatively and had a good course under corticosteroid treatment, with the resolution of clinical, laboratory and imaging findings over the next couple of months. Conclusions. Our patient most probably developed this complication due to a recent SARS-CoV-2 infection. A recent or acute SARS-CoV-2 infection should also be ruled out before considering the etiology as indeterminate when facing a pediatric ALF case. The clinical course of ALF in children is often unpredictable; thus, prompt diagnosis and initiation of conservative treatment may improve the outcome of these patients.
Acute liver failure associated with recent SARS-CoV-2 infection in a pediatric patient – case report
Insuficienţă hepatică acută asociată cu infecţie recentă cu SARS-CoV-2 la un copil – prezentare de caz
First published: 12 aprilie 2022
Editorial Group: MEDICHUB MEDIA
DOI: 10.26416/Pedi.65.1.2022.6336
Abstract
Rezumat
Introducere. Insuficienţa hepatică acută (IHA) la copil este o patologie rară, dar severă, caracterizată prin coagulopatie şi modificări de laborator sugestive pentru injuria hepatică acută, la un pacient fără istoric de afectare hepatică preexistentă. Infecţia cu SARS-CoV-2 (coronavirusul sindromului respirator acut sever 2) provoacă frecvent simptome gastrointestinale la copil, însă afectarea hepatică este de obicei uşoară şi autolimitantă. Prezentare de caz. Prezentăm cazul unui băiat de 5 ani, fără antecedente patologice semnificative, care s-a internat pentru icter, scaune decolorate şi urini hipercrome debutate brusc, asociate cu dureri abdominale şi vărsături. Investigaţiile de laborator au evidenţiat coagulopatie severă (timp de protrombină: 126,9 s, international normalized ratio: 6,14), hipoglicemie, hipoalbuminemie, transaminaze crescute (aspartat aminotransferază: 1254 UI/L, alanin aminotransferază: 1548 UI/L), hiperbilirubinemie directă (bilirubină totală: 27,69 mg/dL, bilirubină directă: 20,74 mg/dL). Cele mai frecvente cauze infecţioase, metabolice, autoimune şi toxice de IHA la copil au fost excluse. S-a ridicat iniţial suspiciunea de boală Wilson, pe baza nivelului scăzut al ceruloplasminei şi al cupruriei crescute, însă valorile s-au normalizat la determinările ulterioare. Ecografia abdominală a relevat ficat hiperecogen, cu dimensiuni crescute, colecist cu pereţi îngroşaţi (14 mm), cu microabcese, fără imagini de calculi şi lichid liber intraperitoneal în cantitate mică. Testul real-time PCR pentru SARS-CoV-2 a fost negativ la internare, însă serologia a evidenţiat anticorpi IgG pozitivi în titru mare (157 AU/ml), cu anticorpi IgM negativi. Pacientul a fost tratat conservativ, iar evoluţia a fost favorabilă sub corticoterapie, cu remiterea modificărilor clinice, paraclinice şi imagistice pe parcursul următoarelor două luni. Concluzii. Pacientul a dezvoltat această complicaţie cel mai probabil în urma infecţiei cu SARS-CoV-2. În faţa unui copil cu IHA, infecţia acută sau recentă cu SARS-CoV-2 ar trebui, de asemenea, exclusă. Evoluţia IHA la copil este deseori imprevizibilă, iar diagnosticul prompt şi iniţierea rapidă a unui tratament conservartiv ar putea conduce la îmbunătăţirea prognosticului.
Introduction
Pediatric acute liver failure (ALF) is a rare but severe condition characterized by coagulopathy and laboratory findings of acute liver injury in a patient without any history of previous hepatic disease(1). In the presence of severe coagulopathy (international normalized ratio [INR]>2), hepatic encephalopathy is not required for establishing a diagnosis of ALF in children(2). The etiology of pediatric ALF varies widely between age groups, with infectious, metabolic, toxic, hematologic and autoimmune diseases being the leading causes. At the same time, a significant number of pediatric ALF cases remain of unknown etiology(3,4). The clinical course of pediatric ALF is often unpredictable, as the disease can rapidly progress towards spontaneous resolution or death in the absence of specific treatment or emergency liver transplantation (LT)(5). While identifying the children with ALF who might benefit from LT remains challenging, timely etiological diagnosis is crucial for improving the management and outcome of these patients(6). Children may develop acute hepatitis and even ALF due to infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(7). Although the coronavirus disease 2019 (COVID-19) in children seems to be less severe than in adults, they may also develop serious complications, including the multisystem inflammatory syndrome in children (MIS-C)(8). Gastrointestinal and hepatic involvement associated with SARS-CoV-2 infection and MIS-C is common in the pediatric population(9). While most children infected with SARS-CoV-2 exhibit mild to moderate liver dysfunction, comprising increased transaminases and cholestasis, severe hepatic involvement has been reported, including ALF(10). This paper reports a child with ALF associated with a recent SARS-CoV-2 infection.
Case report
We report the case of a 5-year-old boy who was transferred to our hospital for further investigation and treatment of ALF. He had no significant medical history and no family history of liver disorders or other chronic conditions. The disease started three weeks before admission to our hospital when the family noticed a sudden onset of jaundice, associated with pale stools, dark urine, abdominal pain and vomiting. The patient presented to a secondary care hospital where the laboratory tests revealed increased transaminases and hyperbilirubinemia. At first, the parents refused the hospitalization, and he was treated at home with hepatoprotective medication. The clinical course was unfavorable, as the patient presented the intensification of jaundice, sleepiness and loss of appetite. Twelve days after the onset of clinical manifestations, he was admitted to another tertiary center with ALF. The initial laboratory tests revealed severe coagulopathy (prothrombin time [PT] 126.9 s, INR 6.14), hypoglycemia, hypoalbuminemia, increased transaminases (aspartate aminotransferase [AST] 1254 IU/L, alanine aminotransferase [ALT] 1548 IU/L), direct hyperbilirubinemia (total bilirubin [TB] 27.69 mg/dL, direct bilirubin [DB] 20.74 mg/dL), hyperammonemia (287 µg/dL), without inflammatory syndrome. The infections with hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus 1,2 (HSV-1,2) and Toxoplasma gondii were excluded based on negative serologies. Real-time polymerase chain reaction (rtPCR) for SARS-CoV-2 was negative. There were no arguments for an autoimmune etiology, and the patient and his family denied the consumption of hepatotoxic drugs and exposure to other toxins. The ceruloplasmin level was decreased (0.141 g/L), and the 24-hour urinary copper excretion was increased (296.96 µg); thus, a diagnosis of Wilson’s disease (WD) was considered. An abdominal ultrasound showed hepatomegaly, increased liver echogenicity and signs of acute acalculous cholecystitis comprising gallbladder mural thickening (14 mm) with parietal microabscesses in the absence of gallstones and free intraperitoneal fluid in a small amount. Additional findings on abdominal computed tomography (CT) scan included intrahepatic biliary dilatation and the presence of pericholecystic fluid. The patient received antibiotic treatment (ampicillin, rifaximin), intravenous steroids (hydrocortisone hemisuccinate), N-acetylcysteine, hepatoprotective agents (arginine-sorbitol, flavonoids), vitamins (B1, B6, B12), zinc supplementation (30 mg/day), lactulose, probiotics, human albumin and diuretic treatment (spironolactone). The management of coagulopathy included the administration of fresh frozen plasma (FFP) and cryoprecipitate. After one week of treatment, the patient showed improvement of clinical state and laboratory parameters, and he was referred to our hospital for further investigations and treatment.
Upon admission to our hospital, the patient exhibited increased irritability, agitation, persistent jaundice, erythematous papular rash on the face, upper and lower limbs, edema of the feet and ankles. The respiratory and cardiovascular examinations were normal, with a heart rate of 71/minute and blood pressure of 101/64 mmHg. Abdominal examination revealed mild abdominal distention, no signs of acute abdomen, hepatomegaly (liver edge palpable at 6 cm below the costal margin), spleen palpable on deep inspiration. Laboratory tests showed decreasing values of transaminases (AST 633 IU/L, ALT 855 IU/L), cholestasis (gammaglutamyl transferase [GGT] 88 IU/L, alkaline phosphatase [ALP] within normal ranges), persistent direct hyperbilirubinemia (TB 17.65 mg/dl, DB 12.73 mg/dl) and improved coagulation parameters (PT 25.6 s, INR 1.93). Further etiological investigations revealed negative serology for hepatitis A virus (HAV), Toxoplasma gondii and HSV-1,2, anti-parvovirus B19 IgM antibodies positive in low titer (1.4 NTU), positive IgG antibodies and negative IgM antibodies for CMV and EBV, anti-SARS-CoV-2 IgG antibodies positive in high titer (157 AU/ml) with negative IgM antibodies. When retaking the medical history, we found out that the patient and his family presented COVID-19 related symptoms two to three weeks before jaundice. Still, none of them was tested for SARS-CoV-2 infection. The inflammatory markers showed increased procalcitonin level (1.03 ng/ml), and normal levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Alpha-1 antitrypsin (A1AT) deficiency was excluded based on normal A1AT levels. The 24-hour urinary copper excretion was within normal ranges. The level of ceruloplasmin increased compared to the first determination (0.165 g/L) and went up to the normal values on subsequent testing, making the diagnosis of WD less probable. Autoimmune etiology was considered improbable, as the serum immunoglobulin G (IgG) levels were normal, and the antinuclear antibodies (ANA), anti-smooth muscle antibodies (SMA) and anti-liver kidney microsomal type 1 antibodies (anti-LKM1) were negative. A diagnosis of hemophagocytic lymphohistiocytosis (HLH) was excluded based on laboratory findings (absence of cytopenia, normal levels of triglycerides, ferritin and soluble interleukin-2), and there were no arguments for other oncologic/hematologic disorders.
Ultrasound reevaluation showed persistent hepatomegaly and liver hyperechogenicity, gallbladder mural thickening in remission (6-7 mm), and no gallbladder stones (Figure 1). No abnormalities were observed on echocardiography. We continued the antibiotic therapy (intravenous ceftazidime for 10 days), associated corticosteroid treatment (hydrocortisone hemisuccinate, followed by oral methylprednisolone 1 mg/kg/day), choleretic agents (ursodeoxycholic acid; UDCA), diuretics (furosemide, spironolactone), calcium and vitamin D supplement. The patient’s clinical and biological parameters improved significantly, and he was discharged from our hospital after 10 days, with recommendations to continue the treatment with oral steroids, UDCA and vitamines.
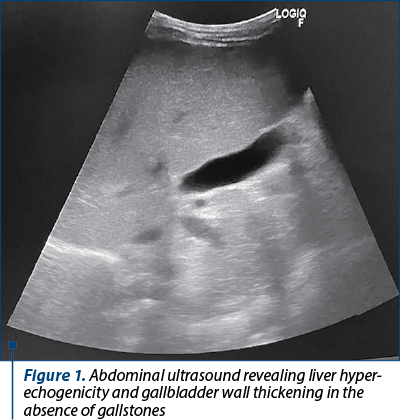
On the second follow-up appointment (two weeks after discharge), the patient presented sinus tachycardia, and the echocardiographic evaluation showed left ventricular hypertrophy with normal left ventricular ejection fraction (LVEF). The N-terminal-prohormone BNP (NT-proBNP) was increased (313.50 pg/mL). He presented high blood pressure (140/90 mmHg), associated with microscopic hematuria and negative proteinuria, indicating a nephritic syndrome. Serum urea and creatinine levels remained normal. Renal ultrasound showed slight bilateral renal enlargement and increased renal echogenicity. The patient received antihypertensive therapy with an angiotensin-converting enzyme (ACE) inhibitor (enalapril), diuretics (furosemide, spironolactone) and beta-blockers (propranolol). Normalizing NT-proBNP and the remission of microscopic hematuria, correlated with normal blood pressure (BP) values, enabled us to stop the antihypertensive treatment after one month.
The laboratory tests revealed favorable hepatic evolution with the normalization of coagulation parameters, albumin level and a significant decrease of transaminases and cholestasis enzymes; hence, the oral methylprednisolone was discontinued gradually. The patient’s outcome was favorable, with the remission of clinical manifestations. At three months following discharge, the laboratory findings showed slightly elevated ALT (51 IU/L) with normal AST level, mild cholestasis (GGT 50 IU/L, normal FA) and normal bilirubin levels.
Discussion
Pediatric ALF is a potentially life-threatening condition and represents one of the main LT indications in children, after biliary atresia, cholestasis and metabolic diseases(11). The etiology of ALF in children is age-dependent and influenced by the economic status of the provenience country(12). Regardless of the child’s age, a significant proportion of ALF cases remain of undetermined etiology, despite extensive evaluation. Studies report that the cause of ALF could not be determined in over 60% of patients aged 1-5 years old(13). Indeterminate pediatric ALF appears to be typically preceded by viral prodrome days to weeks before the onset of symptoms and is associated with higher mortality and LT rates(14).
We presented the case of a 5-year-old boy who developed ALF preceded by a viral prodrome (fever, respiratory symptoms) a couple of weeks before the onset of jaundice. An extensive etiological evaluation was performed, and the most common infectious, metabolic, autoimmune and toxic causes were excluded. WD was initially suspected, based on the low levels of ceruloplasmin and the high 24-hour urinary copper excretion, but the values normalized on the following determinations. Moreover, a low ceruloplasmin level and increased urinary copper excretion can be attributed to the liver failure of any cause(15) . Other biochemical markers have higher sensitivity and specificity for ALF diagnosis due to WD, such as an ALP/TB ratio <4 and AST/ALT>2.2(16). Still, in our patient, these ratios were inconsistent with WD. Fulminant WD often presents with a mild increase in serum transaminases (<500 IU/L) and is highly fatal without emergency LT(15). Our patient had significantly higher transaminases levels and the clinical course was favorable under conservative treatment, making the diagnosis of fulminant WD improbable.
In this case, we could not identify a possible cause of ALF other than the association with anti-SARS-CoV-2 IgG antibodies in high titer, confirming a relatively recent COVID-19 infection. Regardless of the etiology, a nonspecific prodrome is common in pediatric ALF(17). The fact that our patient’s family members also presented respiratory symptoms in the same period, in the setting of an accelerated transmission of SARS-CoV-2 in Romania (during the fourth wave of COVID-19), correlated with the presence of specific IgG antibodies for SARS-CoV-2, advocates for the contribution of SARS-CoV-2 infection to the clinical picture of our case.
It was demonstrated that SARS-CoV-2 affects the respiratory tract and may also cause cardiac, digestive or endocrine symptoms. SARS-CoV-2 related liver injury is most often self-limited and comprises high levels of AST, ALT, bilirubin, GGT, ALP and hypoalbuminemia in severe cases(18). Some of the patients with MIS-C present digestive symptoms that might mimic an acute abdomen; hence, abdominal imaging is often performed before establishing a diagnosis of MIS-C. Gallbladder mural thickening, gallbladder sludge, free intraperitoneal fluid in small amounts, bowel wall thickening and mesenteric lymphadenopathy are common imaging findings in MIS-C patients, and other abnormalities such as hepatosplenomegaly and increased renal echogenicity are found in some cases(9). Acute acalculous cholecystitis associated with SARS-CoV-2 infection has also been reported, but the pathophysiology of the gallbladder and hepatic dysfunction in these patients is not yet fully understood. SARS-CoV-2 enters the host cells via angiotensin-converting enzyme 2 (ACE-2) receptors found in various organs and tissues, including the respiratory tract, liver, gallbladder and kidneys. However, immunological mechanisms, systemic inflammation and cytokine storm may also be involved(19-21). ALF was reported in a SARS-CoV-2 positive pediatric patient with no medical history, whose condition rapidly worsened despite medical support and unfortunately died before receiving an LT(10). Our patient presented an ALF associated with acute acalculous cholecystitis after the acute phase of SARS-CoV-2 infection (negative IgM and positive IgG antibodies), suggesting post-COVID-19 dysregulated immune response as a possible etiology.
Renal involvement in SARS-CoV-2 infection has also been reported in the literature. The pathophysiology is likely multifactorial, intricating direct tropism of the virus for the kidneys and systemic consequences of the infection. Proteinuria, microscopic and macroscopic hematuria, leucocyturia, secondary hypertension and, in more severe cases, acute kidney injury may complicate the clinical course of these patients, even in the absence of chronic kidney disease(22-24). Our patient developed nephritic syndrome in the course of the disease, which rapidly resolved under antihypertensive treatment.
Considering the association of ALF, acute acalculous cholecystitis and nephritic syndrome, on the one hand, and the favorable outcome of our patient under corticosteroid treatment, on the other hand, we suggest that there might be an underlying dysregulation of the immune system determined by SARS-CoV-2 infection. Further research is needed to elucidate the mechanisms behind the multisystemic involvement in SARS-CoV-2 affected pediatric patients.
Conclusions
We conclude that our patient most probably developed this complication due to a recent SARS-CoV-2 infection. The diagnosis of pediatric ALF is based on a high level of suspicion and requires an extensive evaluation to identify the underlying cause. A recent or acute SARS-CoV-2 infection should also be ruled out when facing ALF in children. The clinical course of ALF in children is often unpredictable; thus, prompt diagnosis and the initiation of conservative treatment may improve the outcome of these patients.
Conflict of interests: The authors declare no conflict of interests.
Bibliografie
-
Cochran JB, Losek JD. Acute liver failure in children. Pediatr Emerg Care. 2007;23(2):129–35.
-
Newland CD. Acute liver failure. Pediatr Ann. 2016;45(12):e433–8.
-
Grama A, Aldea CO, Burac L, Delean D, Bulata B, Sirbe C, et al. Etiology and outcome of acute liver failure in children – the experience of a single tertiary care hospital from Romania. Children. 2020;7(12):1–8.
-
Patterson J, Hussey HS, Silal S, Goddard L, Setshedi M, Spearman W, et al. Systematic review of the global epidemiology of viral-induced acute liver failure. BMJ Open. 2020;10(7):e037473.
-
Squires JE, McKiernan P, Squires RH. Acute Liver Failure: An Update. Clin Liver Dis [Internet]. 2018;22(4):773–805. Available from: https://doi.org/10.1016/j.cld.2018.06.009
-
Pop TL, Aldea CO, Delean D, Bulata B, Boghiţoiu D, Păcurar D, et al. The Role of Predictive Models in the Assessment of the Poor Outcomes in Pediatric Acute Liver Failure. J Clin Med. 2022;11(2):1–15.
-
Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute Hepatitis Is a Prominent Presentation of the Multisystem Inflammatory Syndrome in Children: A Single-Center Report. Hepatology. 2020;72(5):1522–7.
-
Siebach MK, Piedimonte G, Ley SH. COVID-19 in childhood: Transmission, clinical presentation, complications and risk factors. Pediatr Pulmonol. 2021;56(6):1342–56.
-
Kanmaniraja D, Kurian J, Holder J, Gunther MS, Chernyak V, Hsu K, et al. Review of COVID-19, part 1: Abdominal manifestations in adults and multisystem inflammatory syndrome in children. Clin Imaging [Internet]. 2021;80(June):88–110. Available from: https://doi.org/10.1016/j.clinimag.2021.06.025
-
Haji Esmaeil Memar E, Mamishi S, Sharifzadeh Ekbatani M, Alimadadi H, Yaghmaei B, Chegini V, Janani S, Mahmoudi S. Fulminant hepatic failure: A rare and devastating manifestation of Coronavirus disease 2019 in an 11-year-old boy. Arch Pediatr. 2020;27:502-5.
-
Pham YH, Miloh T. Liver Transplantation in Children. Clin Liver Dis [Internet]. 2018;22(4):807–21. Available from: https://doi.org/10.1016/j.cld.2018.06.004
-
Berardi G, Tuckfield L, DelVecchio MT, Aronoff S. Differential Diagnosis of Acute Liver Failure in Children: A Systematic Review. Pediatr Gastroenterol Hepatol Nutr. 2020;23(6):501–10.
-
Squires RH, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5).
-
Alonso EM, Horslen SP, Behrens EM, Doo E. Pediatric acute liver failure of undetermined cause: A research workshop. Hepatology. 2017;65(3):1026-1037. doi:10.1002/hep.28944.
-
Socha P, Janczyk W, Dhawan A, Baumann U, D’Antiga L, Tanner S, et al. Wilson’s Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition. 2018;66(2):334-344.
-
Korman JD, Volenberg I, Balko J, Webster J, Schiodt F V, Squires RH, et al. Screening for Wilson disease in acute liver failure: A comparison of currently available diagnostic tests. Hepatology. 2008;48(4):1167–74.
-
Shanmugam Nch, Dhawan A. Acute liver failure in CHildren. Pediatr Hepatol Liver Transplant. 2019;145–53.
-
Wang X, Lei J, Li Z, Yan L. Potential Effects of Coronaviruses on the Liver: An Update. Front Med. 2021;8(September):1–20.
-
Alhassan SM, Iqbal P, Fikrey L, Mohamed Ibrahim MI, Qamar MS, Chaponda M, et al. Post COVID 19 acute acalculous cholecystitis raising the possibility of underlying dysregulated immune response, a case report. Ann Med Surg [Internet]. 2020;60(September):434–7. Available from: https://doi.org/10.1016/j.amsu.2020.11.031
-
Abaleka FI, Nigussie B, Bedanie G, Mohammed A, Galiboglu S. Acute Acalculous Cholecystitis Due to COVID-19, an Unusual Presentation. Cureus. 2021;13(6):6–9.
-
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8.
-
Nadim MK, Forni LG, Mehta RL, Connor MJ, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol [Internet]. 2020;16(12):747–64. Available from: http://dx.doi.org/10.1038/s41581-020-00356-5
-
Pousa PA, Mendonça TSC, Oliveira EA, Simões-E-Silva AC. Extrapulmonary manifestations of COVID-19 in children: a comprehensive review and pathophysiological considerations. J Pediatr (Rio J). 2021;97(2):116-139.
-
Martin SM, Battaglia M, Beaudoin ML. Course of renal involvement in the short term in children with coronavirus disease 2019. Arch Argent Pediatr. 2021;119(6):414–8.
Articole din ediţiile anterioare
Modificările ghidurilor în gastroenterologia şi endoscopia digestivă pediatrică în contextul pandemiei cu SARS-CoV-2
he infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the coronavirus disease 2019 (COVID-19).
Imunoterapia modernă
Imunoterapia specifică (ITS), sau vaccinarea alergenică, este o variantă terapeutică utilizată de medicii alergologi pentru a reduce reactivitatea ...
Evaluarea nutriţională cu particularităţi evolutive la pacienţii cu mucoviscidoză
Mucoviscidoza este cea mai frecventă maladie monogenică, autozomal recesivă, în populațiile de origine caucaziană, cu evoluție cronică, letală, cu ...
Cauză inedită de dispnee obstructivă la copil
Dispneea la sugar și la copilul mic reprezintă un simptom frecvent de adresabilitate într-un serviciu medical. Pentru medic, stabilirea diagnosticu...